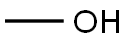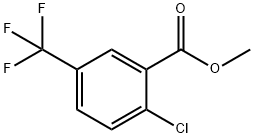
2-CHLORO-5-(TRIFLUOROMETHYL)PHENYL ACETATE synthesis
- Product Name:2-CHLORO-5-(TRIFLUOROMETHYL)PHENYL ACETATE
- CAS Number:26107-79-9
- Molecular formula:C9H6ClF3O2
- Molecular Weight:238.59
122-20-3
384 suppliers
$5.00/10g
657-06-7
192 suppliers
$7.00/1g
77-78-1
303 suppliers
$22.00/25g

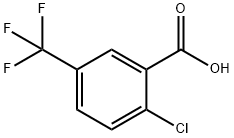
26107-79-9
11 suppliers
inquiry
Yield:26107-79-9 630 g (96%)
Reaction Conditions:
with hydrogenchloride in water;acetone;
Steps:
15.B Step B:
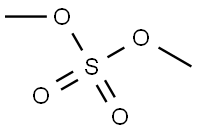
Step B: Preparation of Methyl 2-chloro-5-trifluoromethylbenzoate A sample of 620 g (3.25 moles) of tris-(2-hydroxypropyl)amine was melted on a steam bath. This was added slowly to a solution of 615 g (2.75 moles) of 2-chloro-5-trifluoromethylbenzoic acid and 375 g (282 ml, 2.98 moles) of dimethylsulfate in 750 ml of acetone in a 5 liter flask. (The solution began to boil during the course of the addition and was kept at 30° C. to 40° C. by intermittant cooling in an ice bath.) After addition was complete the solution was boilded on a steam bath for 30 minutes. The hot solution was diluted first with 250 ml water and, after 10 minutes, with 250 ml of 2N HCl and 750 ml of water and allowed to stir overnight. The solution was extracted with two 2 liter portions of dichloromethane and the organic extract was washed with saturated K2 CO3 and water, dried over Na2 SO4 and evaporated to afford 630 g (96%) of methyl 2-chloro-5-trifluoromethylbenzoate as a yellow liquid. TLC (silica gel, 10% CH2 Cl2 -hexane) showed 1 spot (ester at Rf 0.4; this material was pure enough to be used in the next step without distillation. IR (NaCl, neat) 3080, 3000, 2960 (C--H), 1735 (C=O); NMR [CDCl3, (CH3)4 Si] 3.78 (s, 3H, CH3 --), 7.27 (d, 1H, H-3, J3,4 =9), 7.43 (d of d, 1H, H-4, J3,4 =9, J4,6 -2.4), 7.80 (d, 1H, J4,6 -2.5); Mass Spectrum: m/e 240, 238 (1:3, M+), 209, 207 (1:3, M--OCH3), 181, 179 (1:3, M--CO22 CH3). B.p. 95° C. at 4.5 torr.
References:
US5068248,1991,A