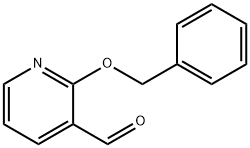
2-Benzyloxy-3-pyridinecarbaldehyde synthesis
- Product Name:2-Benzyloxy-3-pyridinecarbaldehyde
- CAS Number:179257-30-8
- Molecular formula:C13H11NO2
- Molecular Weight:213.23
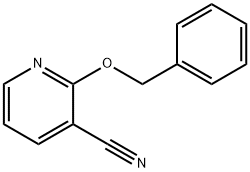
75424-68-9
5 suppliers
inquiry

179257-30-8
8 suppliers
inquiry
Yield:179257-30-8 41%
Reaction Conditions:
Stage #1: 2-(benzyloxy)nicotinonitrilewith diisobutylaluminium hydride in hexane;toluene at -78 - 30; for 1.5 h;
Stage #2: with ammonium chloride in hexane;water;toluene at 1 - 30; for 0.5 h;
Steps:
128 Reference Example 128
To a mixture of benzyl alcohol (32.0 g), 2-chloro-3-cyanopyridine (37.3 g) and N,N-dimethylformamide (200 mL) was added sodium hydride (60%, oil, 12.92 g) under ice-cooling. The reaction mixture was stirred at room temperature for 3 days. Water was added to the reaction mixture and the mixture was extracted with ethyl acetate. The organic layer was washed successively with water and saturated brine, dried over anhydrous magnesium sulfate and concentrated. The obtained residue was subjected to silica gel column chromatography to give 2-(benzyloxy)nicotinonitrile as an oil (39.13 g, 69%) from a fraction eluted with ethyl acetate-hexane (1:6, v/v).<1>H-NMR (CDCl3) delta : 5.52 (2H, s), 6.99 (1H, dd, J=7.6, 5.2 Hz), 7.31-7.52 (5H, m), 7.89 (1H, dd, J=7.6, 2.0 Hz), 8.36 (1H, dd, J=5.2, 2.0 Hz). [0580] To a mixture of 2-(benzyloxy)nicotinonitrile (47.50 g) and anhydrous toluene (100 mL) was dropwise added a solution (1 M, 500mL) of diisobutylaluminum hydride in hexane at -78 DEG C. The reaction mixture was allowed to warm to room temperature with stirring for 1.5 hrs. A saturated aqueous ammonium chloride solution was dropwise added to the mixture and the mixture was stirred at room temperature for 30 min. Ethyl acetate was added to the mixture, and the mixture was further stirred at room temperature for 30 min. Insoluble materials were filtered off. The filtrate was washed with saturated brine, dried over anhydrous magnesium sulfate and concentrated. The obtained residue was subjected to silica gel column chromatography to give 2-(benzyloxy)nicotinaldehyde as an oil (19.71 g, 41%) from a fraction eluted with ethyl acetate-hexane (1:6, v/v).<1>H-NMR (CDCl3) delta : 5.54 (2H, s), 7.04 (1H, dd, J=7.4, 5.2 Hz), 7.26-7.50 (5H, m), 8.14 (1H, dd, J=7.4, 2.0 Hz), 8.40 (1H, dd, J=5.2, 2.0 Hz), 10.45 (1H, s). [0581] To a mixture of potassium t-butoxide (4.52 g) and dimethoxyethane (20 mL) was added a solution (20 mL) of toluenesulfonylmethylisocyanide (4.12 g) in dimethoxyethane at -78 DEG C. Further, a solution (20 mL) of 2-(benzyloxy)nicotinaldehyde (4.12 g) in dimethoxyethane was added to the reaction mixture. The reaction mixture was stirred at -78 DEG C for 1 hr. Methanol (20 mL) was added to the reaction mixture at room temperature and the mixture was heated under reflux for 30 min. To the reaction mixture was added water and the mixture was extracted with ethyl acetate. The organic layer was washed successively with saturated aqueous sodium hydrogencarbonate and saturated brine, dried over anhydrous magnesium sulfate and concentrated. The obtained residue was subjected to silica gel column chromatography to give 2-(2-benzyloxy-3-pyridyl)acetonitrile as an oil from a fraction eluted with ethyl acetate-hexane (1:8, v/v).<1>H-NMR (CDCl3) delta : 3.70 (2H, s), 5.44 (2H, s), 6.95 (1H, dd, J=7.4, 5.0 Hz), 7.32-7.49 (5H, m), 7.71 (1H, dd, J=7.4, 1.8 Hz), 8.17 (1H, dd, J=5.0, 1.8 Hz). [0582] A mixture of 2-(2-benzyloxy-3-pyridyl)acetonitrile (1.0 g) and 10% hydrogen chloride-methanol (30 mL) was stirred at room temperature for 3 days. The reaction mixture was concentrated, and saturated aqueous sodium hydrogencarbonate was added to basify the residue. The mixture was concentrated and a mixed solvent of ethyl acetate and tetrahydrofuran (3:1, v/v) was added to the residue. Insoluble materials were removed by filtration. The filtrate was dried over anhydrous magnesium sulfate and concentrated to give crystals (0.42 g, 56%) of methyl 2-(2-oxo-1,2-dihydro-3-pyridyl)acetate. Recrystallization from ethyl acetate-hexane gave colorless prism crystals. melting point: 183-184 DEG C.
References:
EP1357115,2003,A1 Location in patent:Page/Page column 70
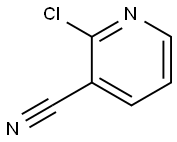
6602-54-6
499 suppliers
$6.00/10g

179257-30-8
8 suppliers
inquiry
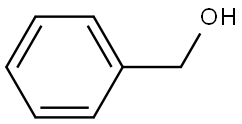
100-51-6
1404 suppliers
$5.00/100g

179257-30-8
8 suppliers
inquiry