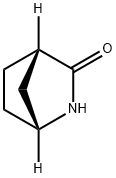
2-Azabicyclo[2.2.1]heptan-3-one, (1R,4S)- synthesis
- Product Name:2-Azabicyclo[2.2.1]heptan-3-one, (1R,4S)-
- CAS Number:134003-02-4
- Molecular formula:C6H9NO
- Molecular Weight:111.14
![(1S,4R)-2-Aza-bicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/130931-83-8.gif)
130931-83-8
170 suppliers
$8.00/100mg
![2-Azabicyclo[2.2.1]heptan-3-one, (1R,4S)-](/CAS/20200119/GIF/134003-02-4.gif)
134003-02-4
12 suppliers
inquiry
Yield:134003-02-4 100%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethyl acetate at 20; for 24 h;
Steps:
3.A
A mixture of (1S)-(+)-2-azabicyclo[2.2.1]hept-5-ene-3-one (10.3 g, 94.4 mmol) in EtOAc (200 mL) and 10% Pd/C (0.5 g) was hydrogenated at RT. After 24 h the reaction mixture was filtered and evaporated leaving behind 10.4 g (100%) of the product that was taken in 250 ml methanol and HCI (12M, 6 ml). The resultant mixture was stirred at RT, until the reaction was complete (72 h). Evaporation of methanol followed by drying under high vacuo, yielded the title compound as an off white solid (16.0 g, 96%). 1H NMR (D2O, 500 MHz): 3.70 (s, 3H), 3.01 (m, 1H), 2.38 (m, 1H), 2.16-1.73 (m, 6H).
References:
WO2005/110409,2005,A2 Location in patent:Page/Page column 38
![2-Azabicyclo[2.2.1]heptan-3-one](/CAS2/GIF/24647-29-8.gif)
24647-29-8
49 suppliers
inquiry
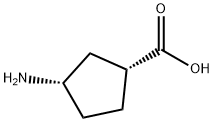
71830-08-5
133 suppliers
$60.00/100mg
![2-Azabicyclo[2.2.1]heptan-3-one, (1R,4S)-](/CAS/20200119/GIF/134003-02-4.gif)
134003-02-4
12 suppliers
inquiry
![2-Azabicyclo[2.2.1]heptan-3-one](/CAS2/GIF/24647-29-8.gif)
24647-29-8
49 suppliers
inquiry
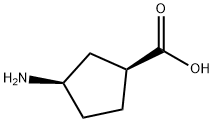
71830-07-4
119 suppliers
$55.00/100mg
![(1S,4R)-2-AZABICYCLO[2.2.1]HEPTAN-3-ONE](/CAS/GIF/134003-03-5.gif)
134003-03-5
18 suppliers
$733.72/250MG
![2-Azabicyclo[2.2.1]heptan-3-one, (1R,4S)-](/CAS/20200119/GIF/134003-02-4.gif)
134003-02-4
12 suppliers
inquiry
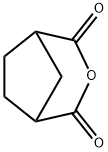
6054-16-6
33 suppliers
$28.60/1mg
![(1S,4R)-2-AZABICYCLO[2.2.1]HEPTAN-3-ONE](/CAS/GIF/134003-03-5.gif)
134003-03-5
18 suppliers
$733.72/250MG
![2-Azabicyclo[2.2.1]heptan-3-one, (1R,4S)-](/CAS/20200119/GIF/134003-02-4.gif)
134003-02-4
12 suppliers
inquiry
![2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/49805-30-3.gif)
49805-30-3
368 suppliers
$5.00/5g
![2-Azabicyclo[2.2.1]heptan-3-one, (1R,4S)-](/CAS/20200119/GIF/134003-02-4.gif)
134003-02-4
12 suppliers
inquiry