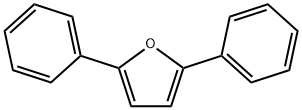
2,5-Diphenylfuran synthesis
- Product Name:2,5-Diphenylfuran
- CAS Number:955-83-9
- Molecular formula:C16H12O
- Molecular Weight:220.27
1H NMR (CDCl?, 500 MHz): δ 7.77 (d, 4H, J = 8.0 Hz), 7.43 (t, 4H, J = 7.5 Hz), 7.29 (t, 2H, J = 7.5 Hz), 6.75 (s, 2H).
13C {1H} NMR (CDCl?, 126 MHz): δ 137.7, 128.8, 128.7, 128.5, 127.5, 80.3.
Using the general batch procedure and 3 (120 mg, 0.50 mmol), PPh? (144 mg, 0.55 mmol), and CBr? (182 mg, 0.55 mmol), 2,5-Diphenylfuran was obtained as a colorless solid after column chromatography (112 mg, 0.50 mmol, 99%); R_f = 0.40 (9/1 hexanes/ethyl acetate).
1H NMR (CDCl?, 500 MHz): δ 7.77 (d, 4H, J = 7.3 Hz), 7.43 (t, 4H, J = 7.5 Hz), 7.29 (t, 2H, J = 7.5 Hz), 6.76 (s, 2H).
13C {1H} NMR (CDCl?, 126 MHz): δ 153.4, 130.9, 128.8, 127.4, 123.8, 107.3.
IR (ν_max, cm?1): 2980, 1662, 1600, 1587, 1508, 1479, 1447, 1386.
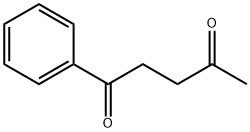
583-05-1
46 suppliers
$39.27/1g

955-83-9
95 suppliers
$65.00/500mg
Yield:955-83-9 94%
Reaction Conditions:
with choline chloride;urea at 80; for 72 h;Paal-Knorr Furan Synthesis;
Steps:
Furan Procedure
General procedure: A solution of dione (1 mmol) in cholinechloride/urea or choline chloride/glycerol (1 mL) was heated to 80 °C andstirred for the designated time. Aftercooling to room temperature, the reaction was diluted with water (2 mL) andextracted with methylene chloride (4 mL).The organic layer was separated, dried, filtered, and concentrated in vacuo to afford the pyrrole productin analytically pure form without further purification.
References:
Handy, Scott;Lavender, Kevin [Tetrahedron Letters,2013,vol. 54,# 33,p. 4377 - 4379] Location in patent:supporting information
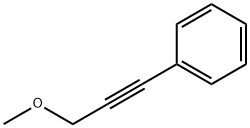
22174-59-0
0 suppliers
inquiry
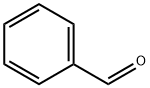
100-52-7
966 suppliers
$20.37/250g
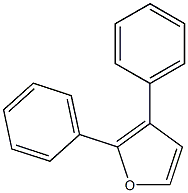
954-55-2
0 suppliers
inquiry

955-83-9
95 suppliers
$65.00/500mg
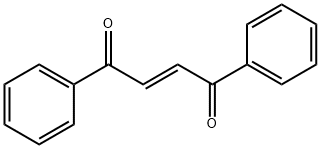
959-28-4
72 suppliers
$27.50/1g

955-83-9
95 suppliers
$65.00/500mg
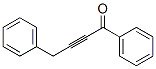
73758-48-2
0 suppliers
inquiry

955-83-9
95 suppliers
$65.00/500mg
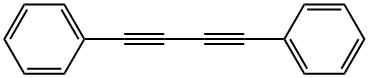
886-66-8
144 suppliers
$24.00/1g

955-83-9
95 suppliers
$65.00/500mg