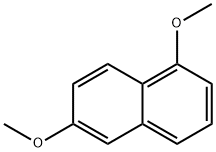
2,5-Dimethoxynaphthalene synthesis
- Product Name:2,5-Dimethoxynaphthalene
- CAS Number:3900-49-0
- Molecular formula:C12H12O2
- Molecular Weight:188.22
Yield: 90%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 2 h;
Steps:
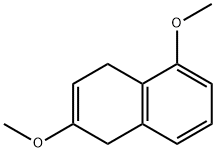
21.1 (1) Synthesis of a Methyl Derivative (Compound 40)
Into a round-bottom flask equipped with a stirring apparatus, 1.6 g (10.0 mmol) of 1,6-dihydroxynaphthalene (Compound 39: produced by Tokyo Chemical Industry Co., Ltd.), and 15 mL of N,N-dimethylformamide (DMF) were added for dissolution, and thrther 14.2 g (100.0 mmol) of methyl iodide (produced by Wako Pure Chemical Industries, Ltd.), and 13.8 g (100.0 mmol) of potassium carbonate (produced by Wako Pure Chemical Industries, Ltd.) were added, and the reaction was carried out at room temperature for 2 hours. Afier completion of the reaction, dichloromethane and water were added, and then an organic layer, obtained by solution separation, was washed with water, and the solvent was removed from the reaction solution by concentration under reduced pressure to obtain 1 .7 g (yield:90%) of a colorless liquid methyl derivative (Compound40).
References:
Wako Pure Chemical Industries, Ltd.;Suzuki, Katsufumi;Tsurumi, Yoshihisa;Kawano, Kei;Imazeki, Shigeaki;Murase, Tetsuji US2017/342031, 2017, A1 Location in patent:Paragraph 0643
32940-13-9
2 suppliers
inquiry

3900-49-0
209 suppliers
$10.00/1g
67-56-1
776 suppliers
$7.29/5ml-f
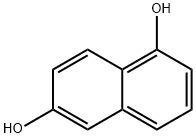
575-44-0
369 suppliers
$7.00/10g

3900-49-0
209 suppliers
$10.00/1g
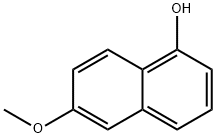
22604-07-5
97 suppliers
$22.00/100mg
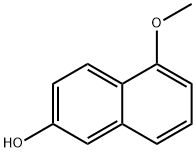
150712-57-5
7 suppliers
inquiry