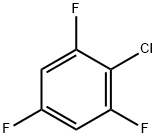
2,4,6-Trifluorochlorobenzene synthesis
- Product Name:2,4,6-Trifluorochlorobenzene
- CAS Number:2106-40-3
- Molecular formula:C6H2ClF3
- Molecular Weight:166.53
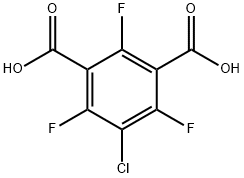
27074-08-4
0 suppliers
inquiry

2106-40-3
105 suppliers
$20.00/1g
Yield: 92%
Reaction Conditions:
in N,N-dimethyl-formamide
Steps:
1 (Preparation of 2-chloro-1,3,5-trifluorobenzene)
EXAMPLE 1 (Preparation of 2-chloro-1,3,5-trifluorobenzene) 60 g of 5-chloro-2,4,6-trifluoroisophthalic acid was added to 90 ml of N,N-dimethylformamide, and the mixture was heated with stirring on an oil bath at 150° C. Carbon dioxide began to evolve at first, and then 2-chloro-1,3,5-trifluorobenzene was distilled off. The distillate was cooled and collected with a Liebig condenser. The generation of carbon dioxide ceased after heating the mixture for about 1 hour, and the mixture was further heated until the distillation of the oily component ceased. The distillate was washed with water, and the oil layer was dried over sodium sulfate to obtain a crude product of 2-chloro-1,3,5-trifluorobenzene in an amount of 36.1 g (yield 92%). The results of NMR analysis of the crude product are as follows: 1 H NMR (CDCl3 /TMS): 6.75 ppm 19 F NMR (CDCl3 /C6 F6): 51.98 ppm (m,2F), 52.66 ppm (m,1F)
References:
SDS Biotech K.K. US5510533, 1996, A
319-88-0
67 suppliers
$25.00/1 g

2106-40-3
105 suppliers
$20.00/1g

319-88-0
67 suppliers
$25.00/1 g
372-38-3
326 suppliers
$9.00/1g
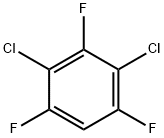
2368-53-8
62 suppliers
$60.00/100mg

2106-40-3
105 suppliers
$20.00/1g
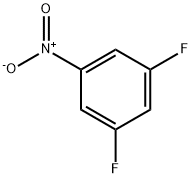
2265-94-3
225 suppliers
$10.00/1g

2106-40-3
105 suppliers
$20.00/1g
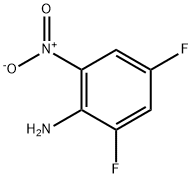
364-30-7
135 suppliers
$10.00/1g

2106-40-3
105 suppliers
$20.00/1g