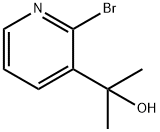
2-(2-BroMo-pyridin-3-yl)-propan-2-ol synthesis
- Product Name:2-(2-BroMo-pyridin-3-yl)-propan-2-ol
- CAS Number:909532-39-4
- Molecular formula:C8H10BrNO
- Molecular Weight:216.0751
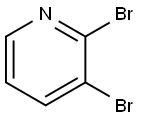
13534-89-9
321 suppliers
$6.00/1g
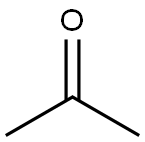
67-64-1
6 suppliers
$19.10/10ml

909532-39-4
14 suppliers
$169.00/250mg
Yield:909532-39-4 48%
Reaction Conditions:
Stage #1: 2,3-dibromopyridinewith isopropylmagnesium chloride in tetrahydrofuran at 20; for 0.75 h;
Stage #2: acetone in tetrahydrofuran at 20;
Steps:
2-(2-Bromopyridin-3-yl)propan-2-ol
To a solution of 2,3-dibromopyridine (5.5 g, 23 mmol) in tetrahydrofuran (77 ml) was added isopropylmagnesium chloride, 2 M in tetrahydrofuran (15 ml, 30 mmol) at room temperature. The reaction mixture was stirred for 45 minutes. Acetone (2.7 g, 3.4 ml, 46 mmol) was added in a quick fashion at room temperature. The reaction mixture was stirred for 16 h and the quenched with 2 M aqueous hydrogen chloride solution (15 ml). The solvent was evaporated. The residue was partitioned between ethyl acetate (100 ml) and water (100 ml). The layers were separated. The aqueous layer was extracted with one 100-ml portion of ethyl acetate. The combined organic layers were washed with one 50-ml portion of saturated ammonium chloride solution, dried over anhydrous sodium sulfate, filtered and concentrated in vacuo. Flash chromatography with w-heptane/ethyl acetate gave the title compound (2.4 g, 48%) as light brown viscous oil. MS m/e: 216, 218 [(M+H)+].
References:
WO2015/91411,2015,A1 Location in patent:Page/Page column 55
109-04-6
530 suppliers
$6.00/25g

67-64-1
6 suppliers
$19.10/10ml

909532-39-4
14 suppliers
$169.00/250mg

67-64-1
6 suppliers
$19.10/10ml

909532-39-4
14 suppliers
$169.00/250mg