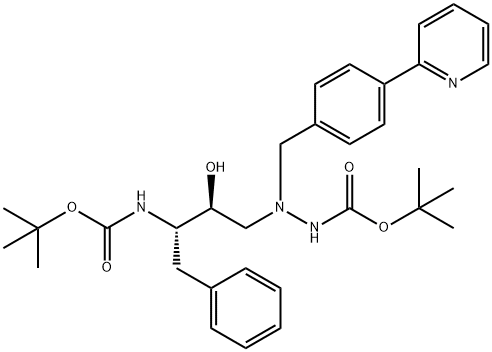
Des-N-(methoxycarbonyl)-L-tert-leucine Bis-Boc Atazanavir synthesis
- Product Name:Des-N-(methoxycarbonyl)-L-tert-leucine Bis-Boc Atazanavir
- CAS Number:198904-86-8
- Molecular formula:C32H42N4O5
- Molecular Weight:562.7
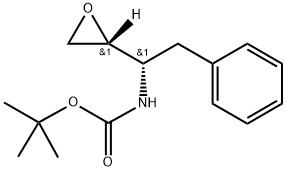
98760-08-8
327 suppliers
$10.00/1g
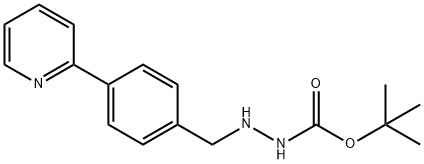
198904-85-7
276 suppliers
inquiry

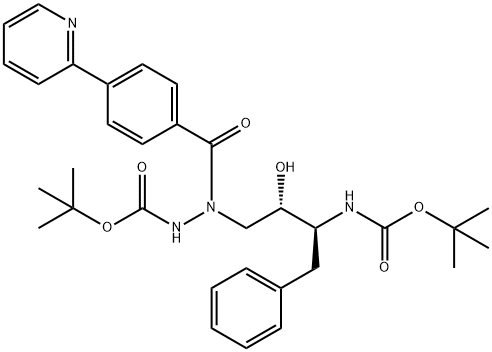
198904-86-8
142 suppliers
inquiry
Yield:198904-86-8 75%
Reaction Conditions:
in isopropyl alcoholHeating / reflux;
Steps:
1
Synthesis of tert-butyl 2-((2S,3S)-3-(tert-butoxyearbonylammo)-2-hydroxy-4-phenylbutyl)-2-(4-(pyndin-2-yl)b enzyl)hydrazinccarboxylate (XV, YIa = YIb = H).; A mixture of tert-butyl (S)-1-((R)-oxian-2-yl)-2-phenylthylcarbamate XIV (1.18 g. 4.48 mmol), XIII, Y1a = Y1b = H (1.23 g, 4.11 mmol) and isopropanol (15 mL) was kept at reflux under nitrogen overnight. The solvent was removed in a rotary evaporator and the residue was purified by chromatography on silica (100 g) with 8:2 dichloromethane/ethyl acetate to give product XV, wherein Y1a = Y1b = II (1.74 g, 75%).
References:
Concert Pharmaceuticals Inc. EP2003120, 2008, A1 Location in patent:Page/Page column 33-34
198905-10-1
1 suppliers
inquiry

198904-86-8
142 suppliers
inquiry
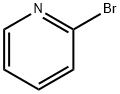
109-04-6
531 suppliers
$6.00/25g

198904-86-8
142 suppliers
inquiry
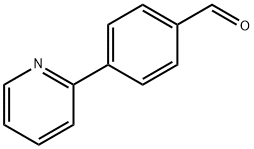
127406-56-8
317 suppliers
$6.00/1g

198904-86-8
142 suppliers
inquiry
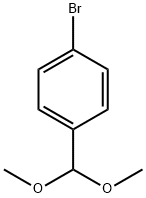
24856-58-4
93 suppliers
$10.00/1g

198904-86-8
142 suppliers
inquiry