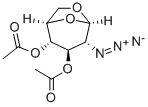
2-Azido-2-deoxy-D-glucopyranose 1,3,4,6-Tetraacetate synthesis
- Product Name:2-Azido-2-deoxy-D-glucopyranose 1,3,4,6-Tetraacetate
- CAS Number:171032-74-9
- Molecular formula:C14H19N3O9
- Molecular Weight:373.32
1078691-95-8
12 suppliers
inquiry
108-24-7
0 suppliers
$14.00/250ML

171032-74-9
50 suppliers
inquiry
Yield:171032-74-9 84%
Reaction Conditions:
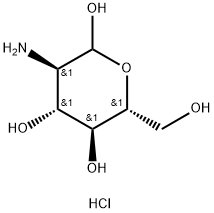
Stage #1:(+)-D-glucosamine hydrochloride with copper(ll) sulfate pentahydrate;triflic azide;triethylamine in water at 20;
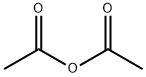
Stage #2:acetic anhydride with pyridine;dmap
Steps:
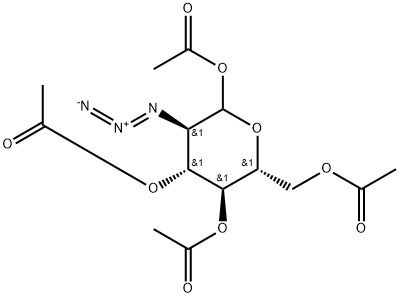
l,3,4,6-Tetra-i?-acetyl-2-azido-2-deoxy-a/p-D-glucopyranose (12).
l,3,4,6-Tetra-i?-acetyl-2-azido-2-deoxy-a/p-D-glucopyranose (12). D-Glucosamine hydrochloride (1.00 g, 4.64 mmol) was dissolved in water (5.0 mL). Triethylamine (937 mg, 9.27 mmol) was added, along with copper sulphate pentahydrate (12 mg, 0.05 mmol). Triflic azide stock solution was then added. The blue mixture was stirred rapidly overnight, and then reduced in vacuo (water bath temperature kept below 20 °C). The resultant green syrup was dissolved in pyridine (10.0 mL), and acetic anhydride (3.0 mL) and DMAP (50 mg) were added slowly. After stirring overmght, the reaction mixture was evaporated to dryness, and the resulting residue was purified by flash chromatography (hexane/EtOAc, 3:2) to yield 12 as a yellow solid (1.44 g, 84%, predominately β anomer); i?f 0.13 (hexane/EtOAc, 4:1); JH NMR data were in agreement with those reported earlier. [221
References:
UNIVERSITY OF MANITOBA;ARTHUR, Gilbert;SCHWEIZER, Frank;SAMADDER, Pranati;XU, Yaozu WO2013/116949, 2013, A1 Location in patent:Page/Page column 32

1078691-95-8
12 suppliers
inquiry

171032-74-9
50 suppliers
inquiry
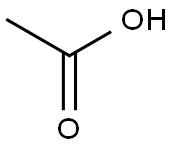
64-19-7
1578 suppliers
$10.00/25ML
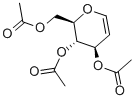
2873-29-2
378 suppliers
$5.00/1g

171032-74-9
50 suppliers
inquiry
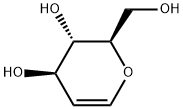
13265-84-4
232 suppliers
$14.00/1g

171032-74-9
50 suppliers
inquiry