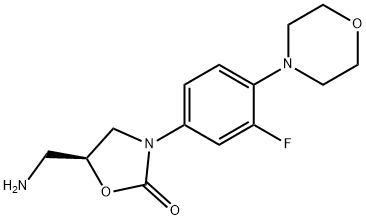
(S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]amine synthesis
- Product Name:(S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]amine
- CAS Number:168828-90-8
- Molecular formula:C14H18FN3O3
- Molecular Weight:295.31
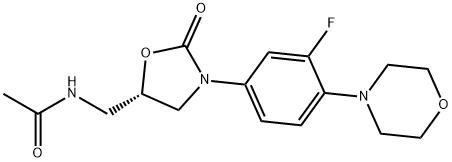
165800-03-3
769 suppliers
$5.00/100mg
![(S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]amine](/CAS/GIF/168828-90-8.gif)
168828-90-8
215 suppliers
$7.00/100mg
Yield:168828-90-8 99%
Reaction Conditions:
with hydrogenchloride in methanol;Reflux;
Steps:
(S)-5-(aminomethyl)-3-(3-fluoro-4-morpholinophenyl)oxazolidin-2-one, DP-60
50 mg of linezolid were dissolved in MeOH (1 mL), then conc. HCl (1 mL) was added and the reaction was heated to reflux for ON. Solvent was evaporated to dryness under vacuum, and then the product was purified by reverse phase column chromatography on prep-HPLC using gradient of (water/acetonitrile/ 0.1 %TFA). Those fractions containing pure product were pooled and lyophilized to give the pure product yellow solid, 99% yield. RP-HPLC (C18): 2 to 50% (ACN/ Water/ 0.1%TFA) in 30 min, tR = 9.391 min, purity of 95%; 1H NMR (399 MHz, Acetone-d6) δ 7.54 (dd, J = 15.0, 2.6 Hz, 1H), 7.20 (ddd, J = 8.9, 2.6, 1.1 Hz, 1H), 7.14 - 6.95 (m, 1H), 5.25 (q, J = 8.9, 8.5 Hz, 1H), 4.44 - 4.23 (m, 2H), 4.09 (dd, J = 9.4, 6.1 Hz, 1H), 3.82 - 3.72 (m, 4H), 3.08 - 2.94 (m, 4H); 19F NMR (376 MHz, Acetone) δ -122.71; LCMS(ESI) C14H18FN3O3, [M+H]+: m/z calcd; 296.1, found; 296.1.
References:
Spaulding, Andrew;Takrouri, Khuloud;Mahalingam, Pornachandran;Cleary, Dillon C.;Cooper, Harold D.;Zucchi, Paola;Tear, Westley;Koleva, Bilyana;Beuning, Penny J.;Hirsch, Elizabeth B.;Aggen, James B. [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 23,p. 5310 - 5321] Location in patent:supporting information
![2-Oxazolidinone, 3-[3-fluoro-4-(4-morpholinyl)phenyl]-5-[[(phenylmethyl)amino]methyl]-, (5S)-](/CAS/20200611/GIF/1236077-61-4.gif)
1236077-61-4
1 suppliers
inquiry
![(S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]amine](/CAS/GIF/168828-90-8.gif)
168828-90-8
215 suppliers
$7.00/100mg
![2-Oxazolidinone, 3-[3-fluoro-4-(4-morpholinyl)phenyl]-5-[(2-propen-1-ylamino)methyl]-, (5S)-](/CAS/20210305/GIF/1215006-07-7.gif)
1215006-07-7
0 suppliers
inquiry
![(S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]amine](/CAS/GIF/168828-90-8.gif)
168828-90-8
215 suppliers
$7.00/100mg
![(R)-5-(Azidomethyl)-3-[3-fluoro-4-(4-morpholinyl)phenyl]-2-oxazolidinone](/CAS2/GIF/168828-84-0.gif)
168828-84-0
121 suppliers
inquiry
![(S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]amine](/CAS/GIF/168828-90-8.gif)
168828-90-8
215 suppliers
$7.00/100mg
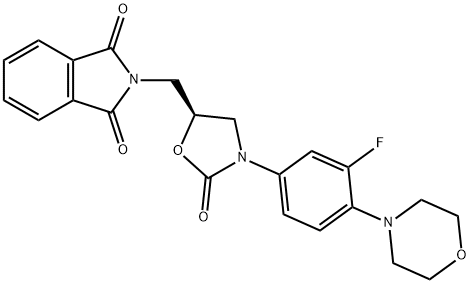
168828-89-5
108 suppliers
$150.00/10mg
![(S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl]methyl]amine](/CAS/GIF/168828-90-8.gif)
168828-90-8
215 suppliers
$7.00/100mg