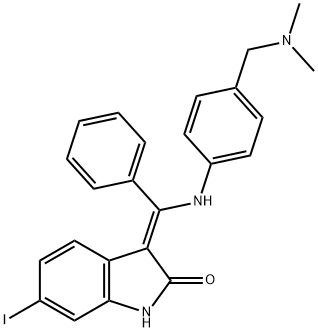
(Z)-3-(((4-((dimethylamino)methyl)phenyl)amino)(phenyl)methylene)-6-iodoindolin-2-one synthesis
- Product Name:(Z)-3-(((4-((dimethylamino)methyl)phenyl)amino)(phenyl)methylene)-6-iodoindolin-2-one
- CAS Number:1537909-08-2
- Molecular formula:C24H22IN3O
- Molecular Weight:495.3554
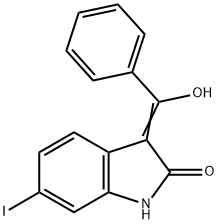
1535204-23-9
8 suppliers
inquiry
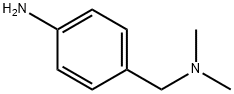
6406-74-2
130 suppliers
$30.00/1g

1537909-08-2
13 suppliers
inquiry
Yield:1537909-08-2 90.6%
Reaction Conditions:
Stage #1: 3-(hydroxy-phenyl-methylene)-6-iodo-1,3-dihydro-indol-2-onewith 1-(Trimethylsilyl)imidazole in toluene at 80 - 90;Inert atmosphere;Large scale;
Stage #2: 4-(dimethylaminomethyl)aniline in toluene; for 10 h;Reflux;Large scale;Temperature;Time;Concentration;
Steps:
2 Step 2, Synthesis of enamine intermediate of formula (II):
Description of the synthesis:In a nitrogen purged vessel, 95 kg (261 ,6 mol) of enol intermediate of formula (IV) are suspended in toluene (315 kg) and heated to an internal temperature of 85°C. Trimethylsilylimidazole (110,1kg) is added at an internal temperature of 80 to 90°C. The addition funnel is flushed with toluene (41 kg) and the reaction mixture is stirred for at least 10mm at an internal temperature of 80 to 90°C. Then a mixture of 4- dimethylaminomethylaniline (47,1kg) and toluene (16kg) is added via the addition funnel. The addition funnel is flushed with toluene (41kg). The resulting reaction mixture is left stirring for 10 hours at reflux (Optionally, the mixture might be left stirring for up to 24 hours at <=80°C). If the content of enol of formula (IV) is smaller than 1,0 area% (H PLC), the reaction mixture is cooled to 55 to 65°C and preheated methanol (413 kg) is added to the reaction mixture in a temperature controlled manner (internal temperature: 55 to 65°C). The suspension is cooled to 15 to 25°C and stirred for at least further 30 minutes (optionally, the mixture might be left stirring for up to 127 hours at room temperature). Then the product is centrifuged and washed with methanol (375kg) and dried at 50°C until <= 0.2% of residual solvent is reached. The product of formula (II) is obtained as a yellow solid in 90,6% yield.
References:
WO2014/9318,2014,A1 Location in patent:Page/Page column 90; 91
919103-45-0
64 suppliers
$111.00/100mg

1537909-08-2
13 suppliers
inquiry
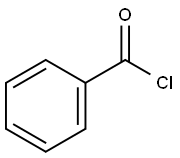
98-88-4
581 suppliers
$10.00/5g

1537909-08-2
13 suppliers
inquiry