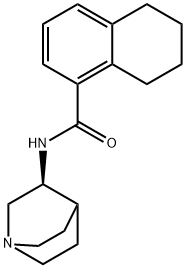
(S)-N-(1-Azabicyclo[2.2.2]oct-3-yl)-5,6,7,8-tetrahydro-1-naphthalenecarboxamide synthesis
- Product Name:(S)-N-(1-Azabicyclo[2.2.2]oct-3-yl)-5,6,7,8-tetrahydro-1-naphthalenecarboxamide
- CAS Number:135729-78-1
- Molecular formula:C18H24N2O
- Molecular Weight:284.4
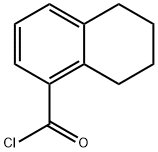
110808-69-0
19 suppliers
$134.31/500mgs:
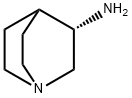
120570-05-0
55 suppliers
$55.00/1
![(S)-N-(1-Azabicyclo[2.2.2]oct-3-yl)-5,6,7,8-tetrahydro-1-naphthalenecarboxamide](/CAS/GIF/135729-78-1.gif)
135729-78-1
86 suppliers
inquiry
Yield: 88.6%
Reaction Conditions:
in toluene at 40 - 65;Inert atmosphere;
Steps:
1.ii.C
Part C: Preparation of N-[(S)-1 -Azabicylco[2.2.2]oct-3-yl]-5,6,7,8- tetrahydro-1-naphthalenecarboxamide (formula III).The acid chloride solution of Part B is combined with the free amine of Part A under a nitrogen atmosphere at 40-600C. The mass is heated to 60-650C and maintained at that temperature for 1-2 hours. Water (300 ml_) is added at 55- 60°C. The mass is made basic by adding 10% NaOH solution (500 ml_). The mass is stirred for 10-20 minutes at 55-600C and the organic layer is separated. The aqueous layer is extracted with toluene (300 ml_) at 55-60°C and the organic layers are combined and washed with water (300 ml_). The organic layer is concentrated under vacuum at 50-55°C until no more solvent distills. Toluene (600 ml_) is charged to the redisue. The mass is cooled to 10-150C and maintained at that temperature for 30-60 minutes. The mass is filtered, washed with toluene (200 ml_), and dried at 65-70°C. Yield: 146.0 g (88.6%). Purity by HPLC: 98.22%.
References:
DR. REDDY'S LABORATORIES LTD.;DR. REDDY'S LABORATORIES, INC.;KATKAM, Srinivas;SAGYAM, Rajeshwar Reddy;SUNKARA, Vishnuvardhan;MUNAGALA, Sridhar;MUTTAVARAPU, Murali Mohan WO2010/56656, 2010, A2 Location in patent:Page/Page column 22
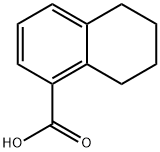
4242-18-6
208 suppliers
$6.00/100mg
![(S)-N-(1-Azabicyclo[2.2.2]oct-3-yl)-5,6,7,8-tetrahydro-1-naphthalenecarboxamide](/CAS/GIF/135729-78-1.gif)
135729-78-1
86 suppliers
inquiry
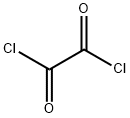
79-37-8
472 suppliers
$17.67/10gm:

4242-18-6
208 suppliers
$6.00/100mg

120570-05-0
55 suppliers
$55.00/1
![(S)-N-(1-Azabicyclo[2.2.2]oct-3-yl)-5,6,7,8-tetrahydro-1-naphthalenecarboxamide](/CAS/GIF/135729-78-1.gif)
135729-78-1
86 suppliers
inquiry