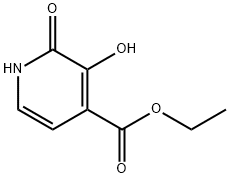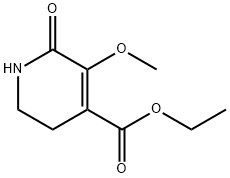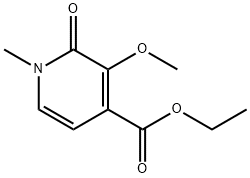
ethyl 3-methoxy-1-methyl-2-oxo-1,2-dihydropyridine-4-carboxylate synthesis
- Product Name:ethyl 3-methoxy-1-methyl-2-oxo-1,2-dihydropyridine-4-carboxylate
- CAS Number:130879-43-5
- Molecular formula:C10H13NO4
- Molecular Weight:211.21
Yield:130879-43-5 63.7%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 80; for 72 h;
Steps:
Intermediate 5. ethyl 3 -methoxy- 1 -methyl-2-oxo- 1 ,2-dihydropyridine-4-carboxylate
Intermediate 5[00166] To a solution of Intermediate 5B (2.0 g, 10.92 mmol) in DMF (15 mL) was added potassium carbonate (4.53 g, 32.8 mmol) and iodomethane (2.72 mL, 43.7 mmol). The reaction was heated at 80 °C for 3 days. The reaction mixture was diluted with DCM, washed with water, dried over MgS04, filtered and concentrated. The residue was dissolved in a small amount of DCM and charged to a 80 g silica gel cartridge which was eluted with a 25 min gradient from 0-100% EtOAc/hexane to give Intermediate 5 (1.47 g, 6.96 mmol, 63.7 % yield) as an off-white solid.HPLC/MS (Method D) RT = 1.2 min, [M + i 212.1 ; 1H NMR (400 MHz,CHLOROFORM-d) δ ppm 7.07 (1 H, d, J=7.28 Hz), 6.33 (1 H, d, J=7.03 Hz), 4.36 (2 H, q, J=7.03 Hz), 4.01 (3 H, s), 3.57 (3 H, s), 1.38 (3 H, t, J=7.15 Hz).
References:
WO2013/48930,2013,A1 Location in patent:Paragraph 00166