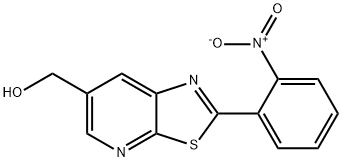
2-(2-Nitrophenyl)-thiazolo[5,4-b]pyridine-6-Methanol synthesis
- Product Name:2-(2-Nitrophenyl)-thiazolo[5,4-b]pyridine-6-Methanol
- CAS Number:1231952-71-8
- Molecular formula:C13H9N3O3S
- Molecular Weight:287.29
![2-(2-Nitrophenyl)-thiazolo[5,4-b]pyridine-6-carboxylic acid Methyl ester](/CAS/20150408/GIF/1231952-70-7.gif)
1231952-70-7
3 suppliers
inquiry
![2-(2-Nitrophenyl)-thiazolo[5,4-b]pyridine-6-Methanol](/CAS/GIF/1231952-71-8.gif)
1231952-71-8
6 suppliers
inquiry
Yield:1231952-71-8 50%
Reaction Conditions:
Stage #1: methyl 2-(2-nitrophenyl)thiazolo[5,4-b]pyridine-6-carboxylatewith lithium aluminium tetrahydride in tetrahydrofuran at -60 - -55; for 12 h;
Stage #2: with water;acetone;sodium sulfite in tetrahydrofuran;
Steps:
1
A solution of methyl 2-(2-nitrophenyl)thiazolo[5,4-δ]pyridine-6-carboxylate (1 eq) in THF was added over 8 h to a mixture of lithium aluminum hydride (LAH) (4.4 eq) in THF maintaining the internal temperature at -55°C. The reaction mixture was stirred an additional 4 h at -600C. Acetone (18 eq) was added followed by sat. aq Na2SO3. The resultant solids were removed by filtration and rinsed with THF. The combined organics were concentrated in vacuo and the crude residue crystallized from CH2Cl2 to give (2-(2-nitrophenyl)thiazolo[5,4-δ]pyridin-6-yl)methanol (50 % yield)
References:
WO2010/71853,2010,A1 Location in patent:Page/Page column 68; 69