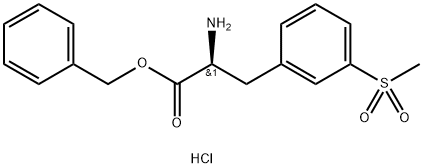
benzyl (S)-2-amino-3-(3-(methylsulfonyl)phenyl)propanoate synthesis
- Product Name:benzyl (S)-2-amino-3-(3-(methylsulfonyl)phenyl)propanoate
- CAS Number:1194550-59-8
- Molecular formula:C17H20ClNO4S
- Molecular Weight:369.86
![L-Phenylalanine,N-[(1,1-dimethylethoxy)carbonyl]-3-(methylsulfonyl)-, phenylmethyl ester](/CAS/20180703/GIF/1289646-78-1.gif)
1289646-78-1
17 suppliers
inquiry

1194550-59-8
130 suppliers
$43.00/1g
Yield:1194550-59-8 94%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;dichloromethane at 0;
Steps:
2; 2A; 2B Example 2
[00121] t-Butylcarbamate (Boc) protection of the amino group of bromophenylalaninewas accomplished, using sodium bicarbonate (3 equivalents), t-butyl dicarbonate (Boc2O, 1.1equivalent) in dioxane and water, to obtain compound 7 in 98% yield. A methyl sulfone functionality was introduced by treating the bromo compound 7 with copper iodide (0.4 equivalents), cesium carbonate (0.5 equivalents), L-proline (0.8 equivalents), and the sodium salt of methanesulfinic acid (3.9 equivalents) in dimethylsulfoxide (DMSO) at 95-100°C for a totalof 9 hours, with two further additions of copper iodide (0.2 equivalents) and L-proline (0.4 equivalents) during that period. Compound 8 was isolated in 96% yield. The carboxylic acid of compound 8 was converted to the benzyl ester, compound 9, in 99% yield, using benzyl alcohol (1 .1 equivalent), dimethylaminopyridine (DMAP, 0.1 equivalent) and N-(3 - dimethylaminopropyl)-N-ethylcarbodiimide (EDC, 1.0 equivalent). The amino group ofcompound 9 is deprotected by adding a 4N solution of HC1 in dioxane to compound 9 at 0°C in methylene chloride. The HC1 salt of the free amino species, compound 10 was isolated in 94% yield.
References:
SARCODE BIOSCIENCE INC.;ZELLER, James, Robert;VENKATRAMAN, Sripathy;BROT, Elisabeth, C.A.;IYER, Subashree;HALL, Michael WO2014/18748, 2014, A1 Location in patent:Paragraph 00120; 00121; 00122; 00123; 00124; 001
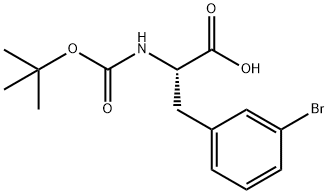
82278-73-7
181 suppliers
$40.00/1G

1194550-59-8
130 suppliers
$43.00/1g
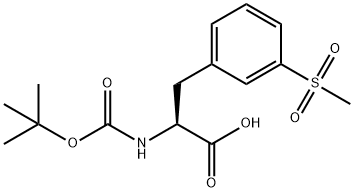
1289646-76-9
51 suppliers
inquiry

1194550-59-8
130 suppliers
$43.00/1g
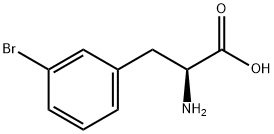
82311-69-1
168 suppliers
$6.00/1g

1194550-59-8
130 suppliers
$43.00/1g