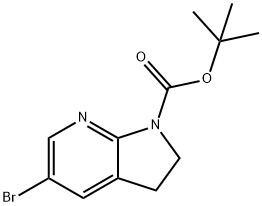
tert-Butyl 5-broMo-2H,3H-pyrrolo[2,3-b]pyridine-1-carboxylate synthesis
- Product Name:tert-Butyl 5-broMo-2H,3H-pyrrolo[2,3-b]pyridine-1-carboxylate
- CAS Number:1111638-13-1
- Molecular formula:C12H15BrN2O2
- Molecular Weight:299.16
24424-99-5
833 suppliers
$13.50/25G
![5-BROMO-2,3-DIHYDRO-1H-PYRROLO[2,3-B]PYRIDINE](/CAS/GIF/115170-40-6.gif)
115170-40-6
148 suppliers
$10.00/100mg
![tert-Butyl 5-broMo-2H,3H-pyrrolo[2,3-b]pyridine-1-carboxylate](/CAS/20150408/GIF/1111638-13-1.gif)
1111638-13-1
21 suppliers
$90.00/50mg
Yield:1111638-13-1 92%
Reaction Conditions:
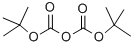
with N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 150; for 1.33333 h;
Steps:
1 Step 1: tert-Butyl-5-bromo-2,3-dihydro-7-aza indole-1-carboxylate
A mixture of 5-bromo-2,3-dihydro-7-aza-indole (0.40 g, 2.0 mmol, prepared from 2,3-dihydro-7-aza-indole according to the procedure described by Graczyk, P. et al., Synthesis of 5-Substituted 7-Azaindoles and 7-Azaindonines, PCT Int. App (2004), WO 2004078757), di-tert-butyl dicarbonate (0.52 g, 2.4 mmol) and N,N-diisopropylethylamine (0.30 mL, 2.2 mmol) in DMF (8 mL) was heated at 150°C for 1 h. Additional di-tert- butyl dicarbonate (0.05 g, 0.23 mmol) and DIEA (0.05 mL, 0.36 mmol) were added, and the mixture was heated at 150°C for an additional 20 min. The solvent was removed in vacuo, and water (10 mL) was added. The mixture was extracted with CH2Cl2, and the organic layer was washed with brine. The organic phase was then dried (Na2SO4), and the solvent was removed in vacuo. The residue was purified by FCC (SiO2; elution with 0- 15% EtOAc/hexanes) to provide 0.55 g (92%) of tert-Butyl-5-bromo-2,3-dihydro-7-aza indole-1-carboxylate.LCMS (Method A): tR= 0.82 min, m/z 299.3/301.2 (M+H)+.
References:
WO2017/214359,2017,A1 Location in patent:Page/Page column 90-91