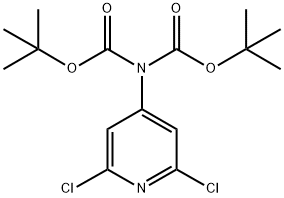
tert-butyl 4-((tert-butoxycarbonyl)amino)-2,6-dichloronicotinate synthesis
- Product Name:tert-butyl 4-((tert-butoxycarbonyl)amino)-2,6-dichloronicotinate
- CAS Number:1044148-88-0
- Molecular formula:C15H20Cl2N2O4
- Molecular Weight:363.24
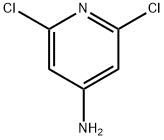
2587-02-2
313 suppliers
$12.40/250mg
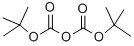
24424-99-5
864 suppliers
$13.50/25G

1044148-88-0
24 suppliers
inquiry
Yield:1044148-88-0 96%
Reaction Conditions:
Stage #1: 2,6-dichloro-[4]pyridylaminewith sodium hexamethyldisilazane in tetrahydrofuran at 0; for 0.5 h;Inert atmosphere;Cooling with ice;
Stage #2: di-tert-butyl dicarbonate in tetrahydrofuran at 20; for 16 h;
Steps:
1 Step 1-Tert-butyl N-tert-butoxycarbonyl-N-(2,6-dichloro-4-pyridyl)carbamate
A solution of 2,6-dichloropyridin-4-amine (28.0 g, 172 mmol, CAS2587-02-2) in THF (400 mL) under nitrogen was cooled to 0° C. with an ice-water bath. To this mixture was added a solution of NaHMDS (1 M, 412 mL) in THF. After the reaction mixture was stirred at 0° C. for 0.5 h, a solution of (Boc)2O (82.5 g, 378 mmol, 86.8 mL) in THF (1.2 L) was added and the ice-water bath was then removed. The reaction mixture was then stirred at rt for 16 hrs. On completion, the reaction mixture was quenched with saturated NH4Cl (1000 mL) and extracted with ethyl acetate (3×500 mL). The combined organic layer was washed with brine (100 mL), dried over anhydrous sodium sulfate, filtered and concentrated in vacuo to give the title compound (42.0 g, 96% yield) as a white solid. 1H NMR (400 MHz, CDCl3) δ 8.98 (s, 1H), 8.34 (s, 1H), 1.63 (s, 9H), 1.53 (s, 9H).
References:
US2019/192668,2019,A1 Location in patent:Paragraph 2642; 2643

14432-12-3
544 suppliers
$5.00/5g

24424-99-5
864 suppliers
$13.50/25G

1044148-88-0
24 suppliers
inquiry