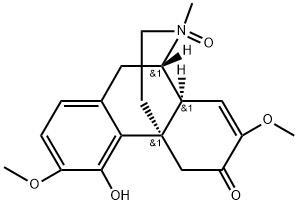
Sinomenine N-oxide synthesis
- Product Name:Sinomenine N-oxide
- CAS Number:1000026-77-6
- Molecular formula:C19H23NO5
- Molecular Weight:345.4
115-53-7
295 suppliers
$39.00/100mg

1000026-77-6
19 suppliers
inquiry
Yield:1000026-77-6 98.7%
Reaction Conditions:
with dihydrogen peroxide at 20; for 24 h;
Steps:
4.1.2.3. 17-Demethyl sinomenine (3)
A solution of 1 (1.32 g, 4 mmol) in 15% H2O2 (20 mL) was allowed to warm to room temperature stirred for 24 h, basified with concentrated ammonia solution (to pH 9-10) and then extracted with CHCl3 (30 mL × 3). The combined organic layers were dried over Na2SO4 and concentrated in vacuum to afford the intermediate oxynitride (1.37 g, 98.7%), which was used in the next step without purification. The intermediate oxynitride (1.37 g, 3.95 mmol) was dissolved in methanol (30 mL) and added with FeSO4·7H2O (2.22 g, 8 mmol) under stirring at 0 °C and then warm to room temperature for 6 h. The solvent was removed and ethylenediaminetetraacetic acid (EDTA) aqueous solution (0.6 M, 40 mL) was added to remove excessive Fe2+ and Fe3+ under stirring for 4 h. The reaction was basified attentively with concentrated ammonia solution (to pH 9-10) and the extracted with CHCl3 (50 mL × 4). The combined organic layers were dried over Na2SO4, filtered and concentrated. Purification by column chromatography (CH2Cl2/MeOH, 15:1-6:1, v/v) afforded the title compound 3 as a gray solid (889 mg, 71.5%). 1H NMR (CDCl3, 300 MHz) δ: 6.65 (d, J = 8.4 Hz, 1H), 6.54 (d, J = 8.4 Hz, 1H), 5.42 (d, J = 2.1 Hz, 1H), 4.33 (d, J = 15.6 Hz, 1H), 3.81 (s, 3H), 3.55 (t, J = 4.2 Hz, 1H), 3.48 (s, 3H), 3.18 (dd, J = 18.0 and 5.7 Hz, 1H), 3.02 (br s, 1H), 2.84 (dd, J = 14.1 and 3.9 Hz, 2H), 2.61 (dt, J = 12.6 and 3.6 Hz, 1H), 2.48 (d, J = 15.6 Hz, 1H), 1.93 (br d, J = 12.6 Hz, 1H). 13C NMR (CDCl3, 75 MHz) δ: 193.8, 152.4, 145.1, 144.8, 130.3, 122.7, 118.4, 114.7, 109.1, 56.0, 54.8, 50.0, 49.6, 45.9, 41.4, 38.9, 36.1, 33.7. Positive ESI-MS m/z: 316 [M+H]+.
References:
Teng, Peng;Liu, Hai-Liang;Deng, Zhang-Shuang;Shi, Zhi-Bing;He, Yun-Mian;Feng, Li-Li;Xu, Qiang;Li, Jian-Xin [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 10,p. 3096 - 3104] Location in patent:experimental part