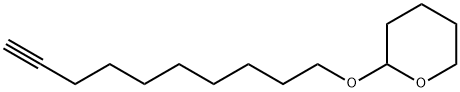
10-(Tetrahydro-2H-pyran-2-yloxy)-1-decyne synthesis
- Product Name:10-(Tetrahydro-2H-pyran-2-yloxy)-1-decyne
- CAS Number:19754-58-6
- Molecular formula:C15H26O2
- Molecular Weight:238.37
Yield:19754-58-6 92%
Reaction Conditions:
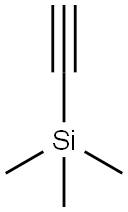
Stage #1: trimethylsilylacetylenewith n-butyllithium in tetrahydrofuran;cyclohexane at -60 - 0; for 0.5 h;
Stage #2: 2-(8-bromooctyloxy)tetrahydropyranwith N,N,N,N,N,N-hexamethylphosphoric triamide in tetrahydrofuran;cyclohexane at -20 - 20; for 19 h;
Stage #3: with tetrabutyl ammonium fluoride in tetrahydrofuran at 20; for 48 h;
Steps:
3.1.12. 6-(1-Heptyl-1H-1,2,3-triazol-4-yl)hexanoic acid (14) (Scheme 3)
General procedure: 3,4-Dihydro-2H-pyran (1.40g, 16.0mmol) was added to stirred solution of 6-bromo-1-hexanol (33a) (2.0g, 11.0mmol) and camphorsulfonic acid (116mg, 5mol%) in Et2O at room temperature overnight. The reaction was basified with satd K2CO3 and extracted into Et2O, washed with brine and dried over Na2SO4. Purification by flash column chromatography; neat CH2Cl2 gave 2-((6-bromohexyl)oxy)tetrahydro-2H-pyran (34a) (2.70g, 95%) as a colourless oil. 1H NMR [DMSO] δ 4.55-4.50 (m, 1H), 3.72 (ddd, J=11.2, 8.1, 3.1Hz, 1H), 3.61 (td, J=9.7, 6.7Hz, 1H), 3.52 (t, J=6.7Hz, 2H), 3.46-3.37 (m, 1H), 3.35-3.28 (m, 1H), 1.84-1.76 (m, 2H), 1.73-1.64 (m, 1H), 1.64-1.54 (m, 1H), 1.54-1.28 (m, 10H). HRMS (ESI+) Calcd for C11H21BrNaO2: 287.0617; (MNa+) 287.0613n-Butyllithium (2.07mL of a 2.0M solution in cyclohexanes, 4.1mmol) was added dropwise to a solution of trimethylsilylacetylene (0.60mL, as a solution in THF, 4.1mmol) in THF (8mL) at -60°C, then the reaction was allowed to warm to 0°C for 30min.12 The reaction was then cooled to -20°C, HMPA (2mL) was added dropwise followed by 34a (1.0g, 3.8mmol). The reaction was stirred for 5h at 0°C and room temperature for an additional 14h, then quenched with satd NH4Cl and extracted into EtOAc, washed with brine and dried over Na2SO4. The crude oil was filtered through a pad of silica with CH2Cl2 and concentrated to dryness to give (by 1H NMR) a 2:1 mixture of trimethyl-(8-((tetrahydro-2H-pyran-2-yl)oxy)oct-1-yn-1-yl)silane (35a) and 2-(oct-7-yn-1-yloxy)tetrahydro-2H-pyran (36a) (1.0g, 98%). This (1.0g, 3.8mmol) was taken up in THF (10mL) and stirred with TBAF (5.6mL as a 1molL-1 solution in THF, 5.6mmol) at room temperature for 48h. The reaction was diluted with EtOAc, and washed with satd NaHCO3, brine, dried over Na2SO4 and concentrated to dryness to give only 36a (740mg, 91%) as a colourless oil.
References:
Marshall, Andrew J.;Lin, Jian-Ming;Grey, Andrew;Reid, Ian R.;Cornish, Jillian;Denny, William A. [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 14,p. 4112 - 4119]
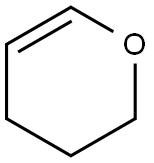
110-87-2
525 suppliers
$10.00/1g
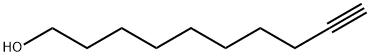
17643-36-6
123 suppliers
$18.00/250mg

19754-58-6
3 suppliers
inquiry

110-87-2
525 suppliers
$10.00/1g

19754-58-6
3 suppliers
inquiry

![1-Octanol, 8-[(tetrahydro-2H-pyran-2-yl)oxy]-, 1-(4-methylbenzenesulfonate)](/CAS/20210305/GIF/108535-80-4.gif)