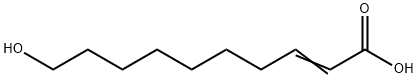
10-Hydroxy-2-decenoic acid synthesis
- Product Name:10-Hydroxy-2-decenoic acid
- CAS Number:765-01-5
- Molecular formula:C10H18O3
- Molecular Weight:186.25
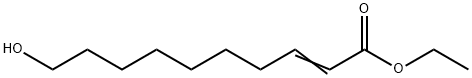
64971-15-9
0 suppliers
inquiry

765-01-5
140 suppliers
inquiry
Yield:765-01-5 71%
Reaction Conditions:
Stage #1:C12H22O3 with sodium carbonate in tetrahydrofuran;water at 65; for 3 h;
Stage #2: with hydrogenchloride;water in tetrahydrofuran; pH=2
Steps:
1.4
4. Saponification Reaction0.60 g (2.81 mmol) of hydroxyester obtained in the preceding step are solubilized in 10 volumes of tetrahydrofurane. 3.4 ml (6.75 mmol) of a 2M soda solution are slowly added. The medium is heated to 65° C. for 3 hours. Once the reaction is completed, the medium is hydrolyzed by adding a 3M hydrochloric acid solution, until pH=2 is obtained. The mixture is dry-concentrated and the aqueous phase is then extracted with ethyl acetate. The organic phases are washed with a NaCl saturated solution, dried on MgSO4, filtered and concentrated in vacuo in order to obtain 0.6 g of crude product.The expected unsaturated hydroxyacid is obtained, as a white solid, by recrystallization from cold acetonitrile, m=0.37 g (71%).The obtained hydroxy acid is a compound of formula (I-2) with n=6: Characterization:TLC: Rf=0.1 (heptane/ethyl acetate 6/4)1H NMR (300 MHz, CDCl3): δ 1.33-1.37 (m, 6H); 1.45-1.49 (m, 2H); 1.55-1.58 (m, 2H); 2.20-2.25 (q, 2H); 3.62-3.66 (t, 2H); 5.79-5.84 (d, J=15.6 Hz, 1H); 7.03-7.10 (dt, J=15.6 Hz, 1H).Mass spectroscopy: [M-Na]+ 209 (calculated 186)Melting point: 62.5° C.+/-1° C.
References:
Pierre Fabre Dermo-Cosmetique US2008/281114, 2008, A1 Location in patent:Page/Page column 11
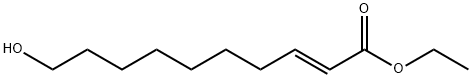
57221-93-9
4 suppliers
inquiry

765-01-5
140 suppliers
inquiry

22054-14-4
4 suppliers
inquiry

765-01-5
140 suppliers
inquiry
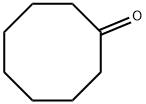
502-49-8
290 suppliers
$8.00/5g

765-01-5
140 suppliers
inquiry
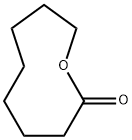
5698-29-3
3 suppliers
inquiry

765-01-5
140 suppliers
inquiry