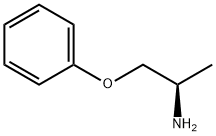
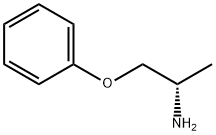
1-Phenoxy-2-propylamine synthesis
- Product Name:1-Phenoxy-2-propylamine
- CAS Number:45972-74-5
- Molecular formula:C9H13NO
- Molecular Weight:151.21
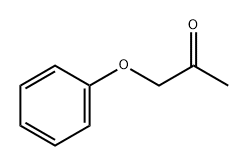
621-87-4
112 suppliers
$43.50/1g
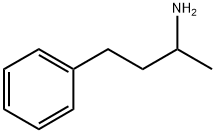
22374-89-6
296 suppliers
$24.00/25g
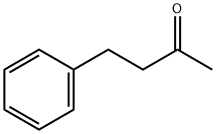
2550-26-7
353 suppliers
$9.00/100g
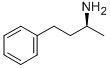
4187-57-9
25 suppliers
$7519.05/50G
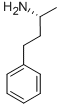
937-52-0
67 suppliers
$215.00/1g

45972-74-5
12 suppliers
inquiry
Yield:> 99 % ee , 2 % ee
Reaction Conditions:
with disodium phosphate heptahydrate;disodium phosphite pentahydrate in toluene at 25; for 72 h;Enzymatic reaction;
Steps:
4.5. PIKAT in Organic Solvents with Immobilized ωTAs
General procedure: In a dark glass vial (2 mL), immobilized EziG3-AsR-ωTA (total mass 22 mg, 10% w w-1 enzymeloading to support material) and hydrate salt pair (Na2HPO3.5H2O/Na2HPO4.7H2O, total mass40 mg, 1:1, w w-1) were suspended in EtOAc (1 mL, at fixed aw of 0.7) and shaken for 15 min (900 rpm,thermomixer). The EziG3-AsR-TA was allowed to sediment and the solvent was removed bypipetting. The EziG3-AsR-ωTA with hydrate salt pair was resuspended in another aliquot of EtOAc,and the above-described process was repeated twice. Next, the EziG3-AsR-ωTA with hydrate salt pairwas washed with toluene (1 mL, at fixed aw of 0.7) and then allowed to sediment, after which thesolvent was removed by pipetting. Finally, toluene (1 mL total reaction volume, at fixed aw of 0.7) wasadded as a reaction solvent. The ketone substrate 1-3a (final concentration: 50 mM) and racemic aminedonor 4-6b (final concentration: 100 mM) were added, and the reaction vials were shaken in an uprightposition (900 rpm, thermomixer) for 72 h at 25 °C. Work-up was performed by separating ketone and amine compounds from each other by extraction. First, the EziG3-AsR-ωTA was left to sediment andthe organic reaction mixture was separated from the biocatalyst by pipetting. Then, naphthalene wasadded as an internal standard to the organic reaction mixture from a concentrated stock solution (50 μLfrom 1Mstock in toluene, final concentration 50 mM). The amine compounds were selectively extractedfrom the toluene reaction phase using an aqueous 2 M HCl solution (400 μL), thereby minimizingthe possible extraction of ketones. Following mixing and centrifugation (14 krpm, 5 min, 4 °C),the two layers were separated and the organic layer was kept separate. The acidic aqueous layer wasre-extracted with toluene (2 x500 μL) to selectively remove potential traces of ketone. The organic layerswere combined and dried over MgSO4 prior to analysis. Then, the acidic aqueous layer-containingthe protonated amine products-was basified using an aqueous solution of either KOH (200 μL of a 10M stock solution) or K2CO3 (for reactions with substrate 2a), and extracted with EtOAc containing50 mM naphthalene as an internal standard (2 x 500 μL). Drying over MgSO4 was performed priorto analysis. Analysis was performed by separately injecting ketone- and amine-containing solutionson GC equipped with an achiral column (see Section 4.6 for details on analytical equipment anddetermination). For the determination of enantiomeric excess, the samples containing amines werederivatized by incubation with 4-dimethylaminopyridine in acetic anhydride (final concentration:5 mg mL-1) for 30 min (170 rpm, RT). Samples were quenched by the addition of water (500 L) andshaken for 30 min (170 rpm, RT). Following centrifugation, the organic layer was dried over MgSO4and analyzed by GC equipped with a chiral column (see Section 4.6 for details on analytical equipmentand determination).
References:
B?hmer, Wesley;Koenekoop, Lucien;Mutti, Francesco G.;Simon, Timothée [Molecules,2020,vol. 25,# 9]
![Carbamic acid, [(1R)-1-methyl-2-phenoxyethyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/20210305/GIF/155768-90-4.gif)
155768-90-4
0 suppliers
inquiry

45972-74-5
12 suppliers
inquiry

621-87-4
112 suppliers
$43.50/1g

109-73-9
455 suppliers
$9.00/1g
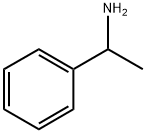
45972-73-4
9 suppliers
inquiry

45972-74-5
12 suppliers
inquiry

71-36-3
1046 suppliers
$14.00/25mL

621-87-4
112 suppliers
$43.50/1g
618-36-0
282 suppliers
$10.00/5mL
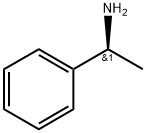
2627-86-3
545 suppliers
$10.60/1gm:
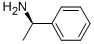
3886-69-9
573 suppliers
$14.00/25G
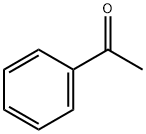
98-86-2
775 suppliers
$9.00/5g

45972-74-5
12 suppliers
inquiry