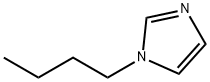
1-Butylimidazole synthesis
- Product Name:1-Butylimidazole
- CAS Number:4316-42-1
- Molecular formula:C7H12N2
- Molecular Weight:124.18
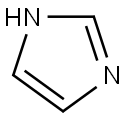
288-32-4
1038 suppliers
$5.00/25g

109-69-3
442 suppliers
$19.00/25mL

4316-42-1
236 suppliers
$10.00/1g
Yield:4316-42-1 78 %Chromat.
Reaction Conditions:
with tetrabutylammomium bromide;sodium hydroxide in water at 100; for 15 h;
Steps:
General Procedure for the Selective N-Benzylation, Allylation, and Alkylation or N,N-Dibenzylation, Allylation, and Alkylation of Primary and Secondary Amines
General procedure: SiO2-CuI (0.1 g,5 mol% Cu) was added to a mixture of amine (0.5 mmol), benzyl chloride, allyl bromide, or n-butyl chloride (0.5 mmol for N-substitution and 1 mmol for N,N-disubstitution), NaOH (2 mmol), and TBAB (0.25 mmol) in a round-bottom flask(25 mL) in water (4mL). The reaction mixture was stirred at 15°C (in the case of N-benzylation, allylation, or alkylation of primary amines,Table 2) or 70-100°C (in the case of N,N-dibenzylation, allylation, or alkylation of primary amines, Table 3), and 100°C(N-benzylation, allylation, and alkylation of secondary amines, Table 4) for an appropriate time. After completion of the reaction (monitored by thin-layer chromatography, TLC), the reaction mixture was triturated with EtOAc (20 mL) and the SiO2-CuI was filtered off. The product was obtained after removal of the solvent under reduced pressure followed by column chromatography or crystallization from EtOAc-petroleum ether.
References:
Shamim, Tahira;Kumar, Vineet;Paul, Satya [Synthetic Communications,2014,vol. 44,# 5,p. 620 - 632]

288-32-4
1038 suppliers
$5.00/25g

109-65-9
563 suppliers
$10.00/10g

4316-42-1
236 suppliers
$10.00/1g

288-32-4
1038 suppliers
$5.00/25g

71-36-3
1071 suppliers
$14.00/25mL

4316-42-1
236 suppliers
$10.00/1g
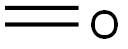
50-00-0
889 suppliers
$10.00/25g

131543-46-9
0 suppliers
inquiry

109-73-9
463 suppliers
$9.00/1g

4316-42-1
236 suppliers
$10.00/1g
