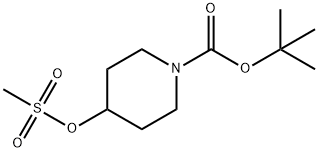
1-Boc-4-methanesulfonyloxypiperidine synthesis
- Product Name:1-Boc-4-methanesulfonyloxypiperidine
- CAS Number:141699-59-4
- Molecular formula:C11H21NO5S
- Molecular Weight:279.35
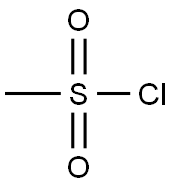
124-63-0
6 suppliers
$10.00/5g
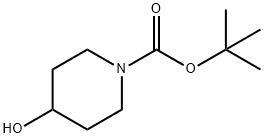
109384-19-2
474 suppliers
$5.00/1g

141699-59-4
233 suppliers
$6.00/1g
Yield:141699-59-4 100%
Reaction Conditions:
with triethylamine in dichloromethane;ethyl acetate
Steps:
14.B Preparation of N-hydroxy-1-(phenyl-methyl)-4-[[4-[4-(trifluoromethoxy)-phenoxy]-1-piperidinyl]sulfonyl]-4-piperidinecarboxamide, monohydrochloride
Part B: Preparation of 4-(methylsulfonyl)hydroxy-1-piperidinecarboxylic acid, 1,1-dimethylethyl ester. To a solution of the BOC piperidine of part A (5.00 g, 24.84 mmol) in dichloromethane (50 mL) at 0° C., was added triethylamine (3.81 mL, 27.32 mmol) followed by methane sulfonyl chloride (2.02 mL, 26.08 mmol). Once the addition was complete the cooling bath was removed. After stirring for two hr the reaction mixture was concentrated in vacuo. The residue was taken up in ethyl acetate, washed with water two times, saturated sodium chloride solution, dried over Na2SO4, filtered, and concentrated in vacuo to provide the mesylate as an off-white solid (7.34 g, >100%).
References:
G. D. Searle & Company US6372758, 2002, B1
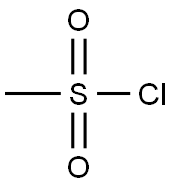
124-63-0
6 suppliers
$10.00/5g

109384-19-2
474 suppliers
$5.00/1g

141699-59-4
233 suppliers
$6.00/1g
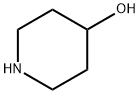
5382-16-1
13 suppliers
$9.00/5 g
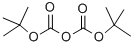
24424-99-5
835 suppliers
$13.50/25G
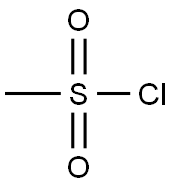
124-63-0
6 suppliers
$10.00/5g

141699-59-4
233 suppliers
$6.00/1g
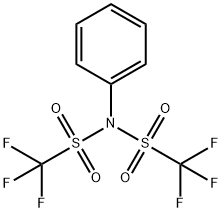
37595-74-7
425 suppliers
$5.00/1g

141699-59-4
233 suppliers
$6.00/1g
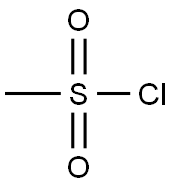
124-63-0
6 suppliers
$10.00/5g

109384-19-2
474 suppliers
$5.00/1g

554-68-7
480 suppliers
$6.00/25g

141699-59-4
233 suppliers
$6.00/1g