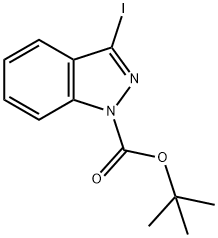
1-Boc-3-iodoindazole synthesis
- Product Name:1-Boc-3-iodoindazole
- CAS Number:290368-00-2
- Molecular formula:C12H13IN2O2
- Molecular Weight:344.15
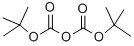
24424-99-5
839 suppliers
$13.50/25G
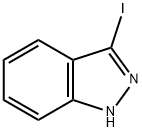
66607-27-0
181 suppliers
$7.00/100mg

290368-00-2
73 suppliers
$29.00/250mg
Yield:290368-00-2 100%
Reaction Conditions:
with triethylamineSonication;
Steps:
tert-Butyl 3-iodo-1H-indazole-1-carboxylate (2a).
A mixture of 3-iodo-1H-indazole (0.2 g, 0.82 mmol), ditert-butyldicarbonate (0.2 g, 0.92 mmol) and triethylamine (1 mL) were put under ultrasonic irradiation for 10 min. The resulted solution was neutralized using HCl 1M and then extracted with dichloromethane (3 × 30 mL). The combined organic layers were dried with anhydrous sodium sulfate and removal of the solvent under vacuum afforded a pure product as a pale yellow crystals.Yield: 100%; m.p.: 93-95 °C; IR (KBr) ν (cm-1): 1728 (C=O); 1150 (C-O); 424 (C-I). 1H-NMR (CDCl3) δ(ppm): 8.09 (1H, d, J = 8.5 Hz, H-7); 7.55 (1H, t, J = 7.8 Hz, H-4); 7.46 (1H, d, J = 7.9 Hz, H-6); 7.33 (1H,t, J = 7.6 Hz, H-5); 1.71 (9H, s, CH3). 13C-NMR δ (ppm): 148.35; 139.59; 130.17; 129.98; 124.21; 121.96;114.56; 102.95; 85.48; 28.18; HRMS calculated for C12H13IN2O2: 344.0022, Found: 344.0016.tert-Butyl 3-iodo-5-nitro-1H-indazole-1-carboxylate (2b). Prepared from 3-iodo-5-nitro-1H-indazole (0.2g, 0.69 mmol), di-tert-butyldicarbonate (0.17 g, 0.78 mmol) and triethylamine (1 mL) to give 0.27 g ofa pale yellow solid. Yield: 100%; m.p.: 144-145 °C; IR (KBr) ν (cm-1): 1744 (C=O); 1528 (NO2tert-Butyl 3-iodo-1H-indazole-1-carboxylate (2a). A mixture of 3-iodo-1H-indazole (0.2 g, 0.82 mmol),di-tert-butyldicarbonate (0.2 g, 0.92 mmol) and triethylamine (1 mL) were put under ultrasonicirradiation for 10 min. The resulted solution was neutralized using HCl 1M and then extractedwith dichloromethane (3 30 mL). The combined organic layers were dried with anhydrous sodiumsulfate and removal of the solvent under vacuum afforded a pure product as a pale yellow crystals.Yield: 100%; m.p.: 93-95 °C; IR (KBr) (cm1): 1728 (C=O); 1150 (C-O); 424 (C-I). 1H-NMR (CDCl3) (ppm): 8.09 (1H, d, J = 8.5 Hz, H-7); 7.55 (1H, t, J = 7.8 Hz, H-4); 7.46 (1H, d, J = 7.9 Hz, H-6); 7.33 (1H,t, J = 7.6 Hz, H-5); 1.71 (9H, s, CH3). 13C-NMR (ppm): 148.35; 139.59; 130.17; 129.98; 124.21; 121.96;114.56; 102.95; 85.48; 28.18; HRMS calculated for C12H13IN2O2: 344.0022, Found: 344.0016.
References:
Vera, Gonzalo;Diethelm, Benjamín;Terraza, Claudio A.;Recabarren-Gajardo, Gonzalo [Molecules,2018,vol. 23,# 8,art. no. 2051]
271-44-3
254 suppliers
$6.00/250mg

290368-00-2
73 suppliers
$29.00/250mg
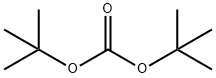
34619-03-9
27 suppliers
inquiry

66607-27-0
181 suppliers
$7.00/100mg

290368-00-2
73 suppliers
$29.00/250mg