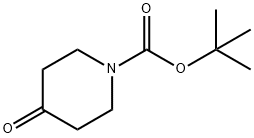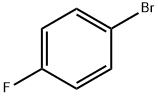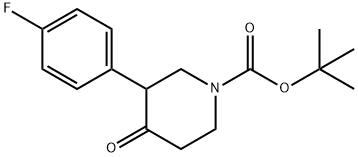
1-BOC-3-(4'-FLUOROPHENYL)-PIPERIDIN-4-ONE synthesis
- Product Name:1-BOC-3-(4'-FLUOROPHENYL)-PIPERIDIN-4-ONE
- CAS Number:632352-74-0
- Molecular formula:C16H20FNO3
- Molecular Weight:293.33
Yield:-
Reaction Conditions:
with sodium t-butanolate;tris-(dibenzylideneacetone)dipalladium(0);2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl in toluene at 20 - 60; for 7.08333 h;
Steps:
12.1
(+/-)-BINAP (0.90 g), sodium tert-butoxide (5.02 g), tris(dibenzylideneacetone)dipalladium (0) (0.55 g) and toluene (200 ml) were mixed under nitrogen atmosphere, and 4-fluorobromobenzene (10.54 g), and then tert-butyl 4-oxo-1-piperidinecarboxylate (8.0 g) were added thereto. The mixture was stirred at room temperature for 5 minutes, and then at 60°C for 7 hours (under nitrogen atmosphere). After cooling to room temperature, the reaction solution was washed with water and saturated brine, dried, and then the solvent was evaporated under reduced pressure. The obtained residue was isolated and purified by silica gel column chromatography (ethyl acetate : hexane = 1 : 4) to obtain tert-butyl 3-(4-fluorophenyl)-4-oxo-1-piperidinecarboxylate (1.46 g) as colorless crystals. 1H-NMR (CDCl3) : δ 1.50 (9H, s), 2.52-2.59 (2H, m), 3.44-3.51 (2H, m), 3.67-3.71 (1H, m), 4.18-4.21 (1H, m), 4.27 (1H, br.), 7.02-7.17 (4H, m).
References:
EP1553084,2005,A1 Location in patent:Page/Page column 48
587-88-2
101 suppliers
$6.00/1g

632352-74-0
12 suppliers
inquiry