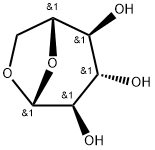
1,6-ANHYDRO-BETA-D-GLUCOPYRANOSE synthesis
- Product Name:1,6-ANHYDRO-BETA-D-GLUCOPYRANOSE
- CAS Number:498-07-7
- Molecular formula:C6H10O5
- Molecular Weight:162.14
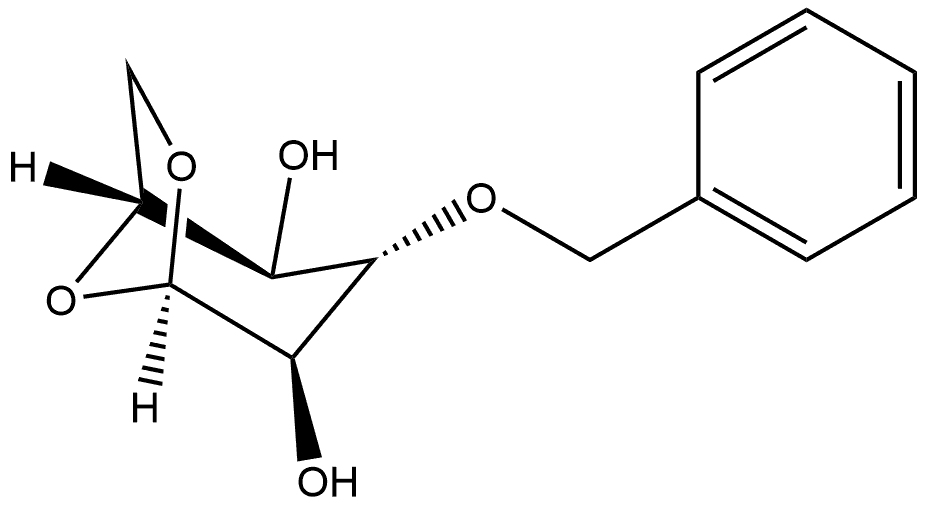
66963-60-8
0 suppliers
inquiry

498-07-7
266 suppliers
$11.00/250mg
Yield:498-07-7 170 g
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol;ethyl acetate at 50; under 3800.26 Torr; for 4 h;
Steps:
1 Preparation of Phenyl-6-O-acetyl-2,3,4-tri-O-benzoyl-1-thio-α-L-idopyranoside
Modified and Optimized Procedure
l,6-anhydro-3-O-benzyl- -L-idopyranose (150g, 0.6 mol) was dissolved in the mixture EtOAc/MeOH (1 : 1, 1200 ml) and hydrogenated over 10% Pd/C (15 g) at 50 °C, 5 atm for 4h. The mixture was cooled to 20 °C, filtered through Celite and evaporated under reduced pressure. The oily residue and DMAP (22 g, 0.18 mol) were dissolved in CH2C12 (400 ml) and Pyridine (404 ml, 5.0 mol) and the resulted mixture was cooled to 0 °C. Benzoyl chloride (276 g, 229 ml, 1.96 mol) was slowly added and the reaction was stirred for 3h at 20 °C (TLC monitoring). The volatiles were evaporated under reduced pressure and the solid residue was portioned between EtOAc (2L) and water (2L). The phases were separated, the organic one was washed with cold 2N aqueous HC1 (700 ml), water (500ml) and 10% aqueous NaHC03 (500 ml), dried over Na2S04, filtered and concentrated under reduced pressure to give ~300g of solid residue. The residue was crystallized from Isopropanol afforded 265g (94%) yield) of the desired tribenzoate as white solid.
References:
OPKO PHARMACEUTICALS, LLC;AHMED, Tahir;GUTMAN, Arie;FEDOTEV, Irina;RUKHMAN, Igor;GROSSMAN, Olga WO2018/35050, 2018, A1 Location in patent:Paragraph 0073
2280-44-6
11 suppliers
inquiry

498-07-7
266 suppliers
$11.00/250mg
1464-44-4
155 suppliers
$24.00/1g

498-07-7
266 suppliers
$11.00/250mg
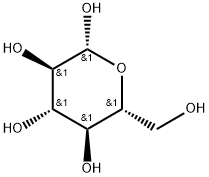
492-61-5
143 suppliers
$26.00/10g

498-07-7
266 suppliers
$11.00/250mg
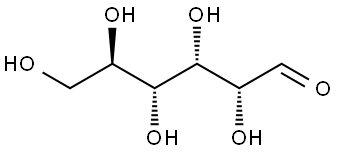
50-99-7
1030 suppliers
$6.00/25g
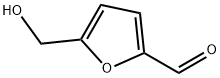
67-47-0
602 suppliers
$5.00/100mg

498-07-7
266 suppliers
$11.00/250mg