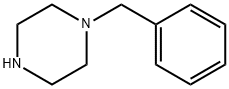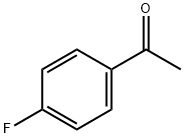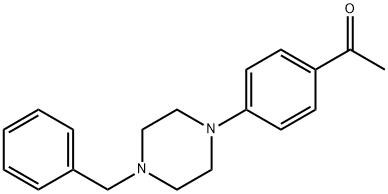
1-[4-(4-BENZYL-PIPERAZIN-1-YL)-PHENYL]-ETHANONE synthesis
- Product Name:1-[4-(4-BENZYL-PIPERAZIN-1-YL)-PHENYL]-ETHANONE
- CAS Number:163733-55-9
- Molecular formula:C19H22N2O
- Molecular Weight:294.39
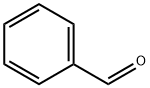
100-52-7
949 suppliers
$21.30/2g
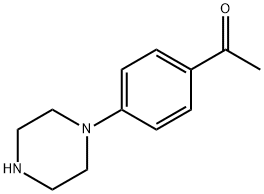
51639-48-6
129 suppliers
$10.60/1gm:
![1-[4-(4-BENZYL-PIPERAZIN-1-YL)-PHENYL]-ETHANONE](/StructureFile/ChemBookStructure3/GIF/CB7350894.gif)
163733-55-9
11 suppliers
inquiry
Yield:163733-55-9 54%
Reaction Conditions:
with sodium tris(acetoxy)borohydride in ethyl acetate at 20;
Steps:
38.A
Example 38: (E)-3-(5-{(E)-3-[4-(4-BenzyI-piperazin-1-yI)-phenyl]-3-oxo- propenyl}-pyridin-2-yl)-N-hydroxy-acrylamide; STEP A; A mixture of 1-(4-piperazin-1-yl-phenyl)-ethanone (500 mg, 2.45 mmol), benzaldehyde (312 mg, 2.94 mmol) and NaBH(OAc)3 (778 mg, 3.67 mmol) in
References:
WO2007/113249,2007,A2 Location in patent:Page/Page column 79-80
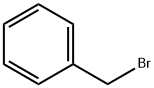
100-39-0
431 suppliers
$10.00/10g

51639-48-6
129 suppliers
$10.60/1gm:
![1-[4-(4-BENZYL-PIPERAZIN-1-YL)-PHENYL]-ETHANONE](/StructureFile/ChemBookStructure3/GIF/CB7350894.gif)
163733-55-9
11 suppliers
inquiry
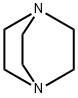
280-57-9
567 suppliers
$5.00/5g
![1-[4-(4-BENZYL-PIPERAZIN-1-YL)-PHENYL]-ETHANONE](/StructureFile/ChemBookStructure3/GIF/CB7350894.gif)
163733-55-9
11 suppliers
inquiry
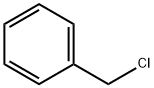
100-44-7
642 suppliers
$13.50/250G
![1-[4-(4-BENZYL-PIPERAZIN-1-YL)-PHENYL]-ETHANONE](/StructureFile/ChemBookStructure3/GIF/CB7350894.gif)
163733-55-9
11 suppliers
inquiry