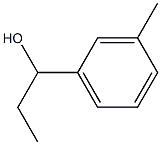
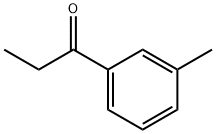
1-(3-methylphenyl)propan-1-ol synthesis
- Product Name:1-(3-methylphenyl)propan-1-ol
- CAS Number:32019-31-1
- Molecular formula:C10H14O
- Molecular Weight:150.22
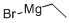
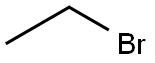
925-90-6
353 suppliers
$12.00/5ml
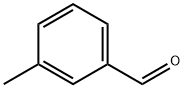
620-23-5
321 suppliers
$5.00/5g

32019-31-1
10 suppliers
$437.00/1g
Yield:32019-31-1 99%
Reaction Conditions:
in tetrahydrofuran;diethyl ether at 0 - 20; for 1.16667 h;Inert atmosphere;
Steps:
21.1 Step 1: l-(i-Tolyl)propan-l-ol
Step 1: l-(i-Tolyl)propan-l-ol To a solution of ethylmagnesium bromide (3.0 M in diethyl ether) (8,30 mL, 24.9 mmol) in THF (8.5 mL) under N2 at about 0 °C was added dropwise a solution of 3-methyibenzaldehyde (1.47 mL, 12.5 mmol) in THF (8.5 mL). The mixture was allowed to stir at about 0 °C for about 10 min. The ice bath was removed and the reaction mixture stirred for about lh at ambient temperature. The reaction mixture was cooled to about 0 °C and quenched slowly by dropwise addition of sat. aq. H4CI. The solvent was partially removed under reduced pressure. EtOAc (30 mL) and water (15 mL) were added. The organic layer was separated and the aqueous layer was back extracted with EtOAc (20 mL). The combined organic layers were washed with sat. aq. NaCI (15 mL), dried over MgS0 , filtered and concentrated under reduced pressure to give the title product (1.87 g, 99%); NMR (400 MHz, DMSO-d6) δ 7.40 - 6.72 (m, 4H), 5.01 (dd, J = 4.4, 0.8 Hz, 1H), 4.42 - 4.34 (m, IH), 2.29 (s, 3H), 1.67 - 1 .50 (m, 2H), 0.81 (t, .7 = 7.4 Hz, 3H).
References:
WO2016/168633,2016,A1 Location in patent:Page/Page column 83-84
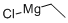
2386-64-3
215 suppliers
$45.00/5ml

620-23-5
321 suppliers
$5.00/5g

32019-31-1
10 suppliers
$437.00/1g
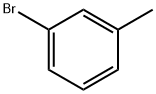
591-17-3
256 suppliers
$10.00/10g
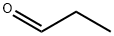
123-38-6
443 suppliers
$14.00/5mL

32019-31-1
10 suppliers
$437.00/1g
51772-30-6
130 suppliers
$45.00/50mg

32019-31-1
10 suppliers
$437.00/1g