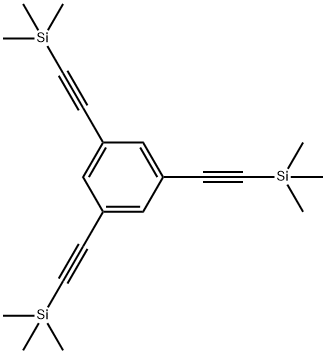
1,3,5-tris((trimethylsilyl)ethynyl)benzene synthesis
- Product Name:1,3,5-tris((trimethylsilyl)ethynyl)benzene
- CAS Number:18772-58-2
- Molecular formula:C21H30Si3
- Molecular Weight:366.72
Yield:18772-58-2 99%
Reaction Conditions:
Stage #1: 1,3,5-Tribromobenzenewith bis-triphenylphosphine-palladium(II) chloride;copper(l) iodide;triethylamine;triphenylphosphine at 50; for 0.25 h;Sonogashira Cross-Coupling;
Stage #2: trimethylsilylacetylene at 90; for 48 h;Sonogashira Cross-Coupling;
Steps:
Compound A:
Freshly dried triethylamine (50 mL) was distilled into a flame-dried 100 mL two-necked round-bottomed flask containing 1,3,5-tribromobenzene (2 g, 6.35 mmol), Pd(PPh3)2Cl2 (223 mg, 0.05 mmol), PPh3 (41.66 mg, 0.03 mmol) and CuI (48 mg, 0.04 mmol). The solution was refluxed at 50 °C for 15 min and trimethylsilylacetylene was added. The reaction mixture was refluxed for 48 h at 90 °C after which triethylamine was fully removed on a rotary evaporator. The black solid was chromatographed on a silica gel column using hexanes to give a light yellow powder as the expected product. Isolated yield: 2.35 g. 1H NMR (CDCl3, 400 MHz): (ppm) 7.49 (s, 3H, H1), 0.24 (s, 27H, H2). 13C{1H} NMR (CDCl3, 100 MHz): (ppm) 135.35, 124.14, 103.62, 96.02, 0.31.
References:
Adeyemo, Aderonke Ajibola;Mukherjee, Partha Sarathi [Beilstein Journal of Organic Chemistry,2018,vol. 14,p. 2242 - 2249] Location in patent:supporting information
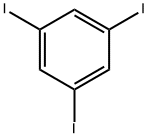
626-44-8
75 suppliers
$36.00/100mg
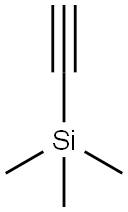
1066-54-2
478 suppliers
$9.00/10g

18772-58-2
19 suppliers
inquiry

1066-54-2
478 suppliers
$9.00/10g

18772-58-2
19 suppliers
inquiry
![Indium, tris[(trimethylsilyl)ethynyl]-](/CAS/20211123/GIF/250643-77-7.gif)