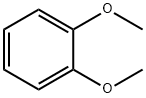
Research Progress on the Synthesis of 1,2-dimethoxybenzene
Jan 23,2025
Introduction
1,2-dimethoxybenzene(Figure 1) is widely used in medicine, pesticides and chemical synthesis. In the field of medicine, 1,2-dimethoxybenzene can be used to synthesize isoboride (a drug used to treat angina and arrhythmia) and tetrahydropalmatine (a hypnotic analgesic drug). It can also detect lactate levels in the blood and measure glycerol; In the field of pesticides, 1,2-dimethoxybenzene is a key intermediate for the synthesis of the fungicide enoxymorpholine. In the field of chemical synthesis, various aryl derivatives can be generated through acylation reactions of 1,2-dimethoxybenzene. For example, 3,4-dimethoxyacetophenone can be used to synthesize insecticides and papaverine (anti spasmodic drugs), while 3,4-dimethoxybenzophenone can be used to synthesize secondary alcohols. 1,2-dimethoxybenzene can also be directly synthesized into methyl vanillin, also known as resveratrol, through the Vilsmeier reaction in one step. This product is widely used in industries such as food, spices, pharmaceutical intermediates, and fine chemical synthesis.

The main synthesis methods of 1,2-dimethoxybenzene include o-aminoanisole method , guaiacol method and catechol method. The o-aminoanisole method and guaiacol method have obvious disadvantages, which limit their application.The catechol method is an ideal choice for industrial synthesis of o-phenylenediol due to its simple operation, easy availability of raw materials, mild reaction conditions and environmental friendliness.According to the different methylating agents, there are several methods for preparing 1,2-dimethoxybenzene by catechol method. When methyl chloride is used as a methylating agent, the yield is high,but the control requirements of reaction conditions are high, and the product separation process is complicated. Dimethyl sulfate is a methylating agent with high reaction activity ,which is suitable fo batch production, but the raw materials are highly toxic,and the process will produce sulfuric acid, which is corrosive to equipment and operators ,and a large amount of wastewater is produced, polluting the environment. When dimethyl carbonate is used as a green methylating agent, no alkali is required, and the process is environmentally friendly, but the choice of catalyst is crucial. When methanol is used as a methylating agent,the cost is low and the post-processing is simple,but the reaction activity is low, and a suitable catalyst needs to be selected to improve the selectivity of 1,2-dimethoxybenzene.[1]
Synthesis methods of 1,2-dimethoxybenzene
o-aminoanisole method
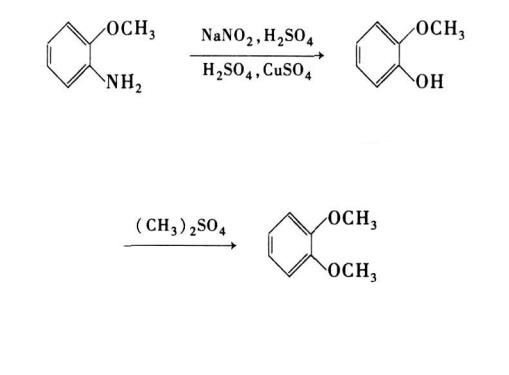
1,2-dimethoxybenzene was prepared from o-anisidine by diazotization hydrolysis and methylation by Zhang etal. (Figure 2)[2]. The optimal reaction conditions were as follows: n(o-anisidine): n(sulfuric acid): n(sodium nitrite)=1.0:3.0:1.01, n(o-hydroxy methylphenate): n(dimethyl sulfate)=l:1.4, the diazotization temperature was 10~15℃, the acid concentration and temperature in process of hydrolysis were 40% and 110℃respectively, the methylation temperature was 80℃, the reaction lasted 3h and the total yield was 84.8%.This method still has its limitations, including high energy consumption, long processes, and severe equipment corrosion. During the production process, there will be the production of highly toxic substances and a significant amount of emissions such as exhaust gas, waste liquid, and waste residue.[2]
Guaiacol method
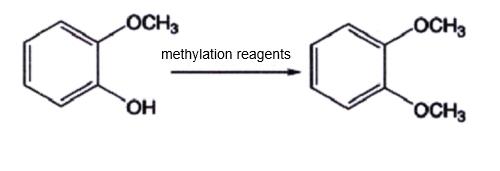
Guaiaco can be used with methylation reagents to prepare 1,2-dimethoxybenzene under the action of catalysts. The synthesis route is shown in Figure 3. Under the action of appropriate methylation reagents and catalysts, guaiacol can be directly synthesized into 1,2-dimethoxybenzene through a one-step reaction. This method has a high yield and is an ideal preparation method. However, as a raw material for the synthesis of 1,2-dimethoxybenzene, guaiacol was mostly extracted from natural guaiacol resins in the early stages. The extraction process is complex, resulting in relatively low production and inability to meet industrial demand.
Catechol method
Pyrocatechol can react with different methylation reagents to produce 1,2-dimethoxybenzene, which is synthesized in two steps. The synthesis route is shown in Figure 4. It is worth mentioning that the reaction between the generation of guaiacol in the first step and the generation of1,2-dimethoxybenzene in the second step is a competitive relationship. Therefore, in order to improve the yield of 1,2-dimethoxybenzene, the selection of catalyst is crucial. This method has the advantages of easy availability of raw materials, mild reaction conditions, and simple operation, making it suitable for industrial production.
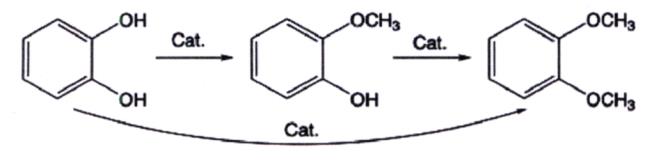
According to different methylation reagents, there are several types:
(1)Chloromethane, as a methylation reagent, is used to prepare 1,2-dimethoxybenzene through Williamson condensation reaction under the action of phase transfer catalyst, with high yield. However, its shortcomings include high requirements for reaction condition control, complex product separation process in the later stage, and difficult purification.[3]
(2)As a highly reactive methylation reagent, dimethyl sulfate is suitable for mass production. It cannot be ignored that the raw material is highly toxic. During the reaction process, sulfuric acid will be produced, posing a corrosive risk to equipment and operators, while accompanied by a large amount of wastewater discharge polluting the environment.
(3)As a green methylation reagent, dimethyl carbonate can achieve a raw material conversion rate of over 96% under the action of different catalysts. The reaction process does not require the participation of alkali and is environmentally friendly and harmless.
(4)Methanol, as a methylation reagent, has low cost and simple post-treatment. However, its reaction activity is low. Therefore, it is necessary to choose a suitable catalyst to improve the selectivity of 1,2-dimethoxybenzene.
References
[1]Xu MJ,Wei JB,etal. Research Progress on the Synthesis of 1,2-dimethoxybenzene[J]. Shandong Chemical Industry,2024,53(21):122-125.
[2]Zhang ZD,Gong GZ,etal.Preparation of 1,2-Dimethoxybenzene[J].Fine and specialty chemicals,2007,(24):16-18.
[3]Zhang HB, Guo JP. Improvement on synthesis of 1,2-Dimethoxybenzene[J].China Chemicals,2005,(03):15-16.
- Related articles
- Related Qustion
Hydrocortisone acetate, a synthetic derivative of the natural steroid hormone hydrocortisone, is widely used in the pharmaceutical and healthcare industries.....
Apr 10,2025Chemical ReagentsEstrone is the second most common type of estrogen produced by during the childbearing years.....
Jan 24,2025API1,2-Dimethoxybenzene
91-16-7You may like
1,2-Dimethoxybenzene manufacturers
- 1,2-Dimethoxybenzene
-

- $6.00 / 1kg
- 2025-04-14
- CAS:91-16-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month
- 1, 2-Dimethoxybenzene
-

- $120.00 / 1kg
- 2025-04-14
- CAS:91-16-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- 1,2-Dimethoxybenzene
-

- $0.00 / 1KG
- 2025-04-11
- CAS:91-16-7
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month