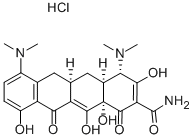
Minocycline hydrochloride: a broad-spectrum antibiotic
Mar 14,2025
Introduction
Minocycline hydrochloride is a tetracycline antibiotic with excellent absorption and tissue penetration that is used for several bacterial infections as well as treatment of acne. Minocycline hydrochloride can cause both an acute hepatitis-like syndrome occurring within 1 to 3 months of starting therapy or a more insidious chronic hepatitis with autoimmune features typically after long term treatment.

Background
Minocycline hydrochloride (min" oh sye' kleen) is a semisynthetic derivative of tetracycline that has excellent oral absorption and wide tissue penetration. Like other tetracyclines, Minocycline hydrochloride is believed to act by binding to bacterial ribosomes and inhibiting protein synthesis. It has a broad spectrum of activity against both gram positive and gram negative organisms. Minocycline hydrochloride was approved for use in the United States in 1971 and it continues in wide use with several million prescriptions being filled yearly. Current indications are for therapy of susceptible infections, including gonorrhea, syphilis, non-gonococcal urethritis, Chlamydial infections, cholera, leprosy, and the meningococcal carrier state. Perhaps the major use of Minocycline hydrochloride is chronic use for treatment of acne and suppression of staphylococcal bacterial flora that contribute to it. It is available in multiple generic forms as capsules or tablets of 50, 75 or 100 mg. For acute infections, Minocycline hydrochloride is recommended in doses of 100 mg every 12 hours for 5 to 15 days, often after an initial loading dose of 200 mg. For therapy of acne, doses of 50 mg once to three times daily are recommended. It is also available in extended release formulations for daily dosing and as a solution or powder for intravenous use. Commercial names of Minocycline hydrochloride include Minocin, Dynacin, Myrac, and Apo-, Novo- and PMS-Minocycline hydrochloride. Common side effects include nausea, diarrhea, gastrointestinal upset, headache, dizziness, visual blurring, skin rash and hypersensitivity reactions.[1]
Hepatotoxicity
Minocycline hydrochloride therapy is associated with two forms of clinically apparent liver injury, an acute hepatitis-like syndrome that arises within 1 to 3 months of starting therapy and a chronic hepatitis-like syndrome typically with autoimmune features that occurs with long term therapy, sometimes after several years of use. There is some overlap between these two presentations of Minocycline hydrochloride hepatotoxicity, both are associated with a hepatocellular pattern of serum enzyme elevations and both can be associated with autoantibodies and immunological features. Minocycline hydrochloride has been linked to cases of an acute hepatitis with jaundice that typically arises within a few weeks or months of starting therapy. The enzyme elevations are typically hepatocellular and resemble acute viral hepatitis. Immunoallergic features are common and may be prominent with fever, rash and eosinophilia and some cases with facial edema, lymphoadenopathy and lymphocytosis that may resemble acute mononucleosis. The liver injury is usually self limited with complete resolution within 1 to 2 months of stopping. Some patients have autoimmune markers and these also improve upon stopping Minocycline hydrochloride.
Minocycline hydrochloride has also been linked to cases of chronic hepatitis with or without jaundice that typically arise during long term therapy, sometimes after years of use. The most common presentation is with an autoimmune hepatitis-like syndrome that can be severe and even fatal, particularly if Minocycline hydrochloride is not stopped promptly. Patients can present acutely with jaundice and fatigue or chronically with insidious onset of fatigue, joint aches and jaundice, usually after 6 months to many years of therapy. A hepatocellular pattern of enzyme elevations is typical with ALT levels ranging from 3- to 20-fold elevated depending upon the severity and duration of the injury. Autoantibodies are usually present, typically antinuclear antibody (ANA) at titers of >1:160. In some cases, ANA may initially be negative, arising later as the disease progresses or starts to improve. Immunoglobulins are also usually elevated and liver biopsy demonstrates changes typical of autoimmune hepatitis with active interface hepatitis, spotty eosinophilic necrosis and portal infiltrates rich in lymphocytes and plasma cells. Other immunologically mediated syndromes associated with Minocycline hydrochloride use include a serum sickness like syndrome (generally within 3 to 12 weeks of starting), a lupus-like syndrome and hemolytic anemia (the latter two with chronic therapy). Liver injury can accompany these other autoimmune conditions, but is generally anicteric, mild and rapidly reversible.[2]
Mechanism of Injury
The cause of the liver injury associated with Minocycline hydrochloride use is probably immunological, mediated by autoimmune reactions against liver cells or which adducts present in the liver. Patients with Minocycline hydrochloride induced liver injury have been reported to have an increased frequency of the rare HLA, but most cases lack this allele and clinical features are not different in those with or without the HLA association.
Outcome and Management
The acute liver injury attributed to Minocycline hydrochloride is usually self-limited in course, although fatal examples have been reported. In addition, the autoimmune hepatitis like syndrome caused by Minocycline hydrochloride can be severe, and fatal outcomes have been described. Most cases, however, resolve slowly with withdrawal of the agent. Corticosteroids are often used to treat Minocycline hydrochloride induced liver injury, largely because it resembles spontaneous autoimmune hepatitis and responds rapidly to immunosuppressive therapy. The effectiveness of corticosteroids in speeding or insuring recovery from Minocycline hydrochloride induced autoimmune hepatitis has not been proven, but anecdotal cases suggest a fairly dramatic effect. If corticosteroids are used, the dose should be rapidly reduced and the medication should be discontinued within 3 to 6 months. Furthermore, patients should be followed carefully after withdrawal of corticosteriods. Recurrence of hepatitis after withdrawal should suggest that the autoimmune hepatitis was unrelated to the Minocycline hydrochloride or that it brought out the autoimmune diathesis that is self-sustaining. Autoimmune hepatitis not caused by medications usually requires long term if not life long immunosuppressive therapy. There is no information about possible cross sensitivity to hepatic injury among the various tetracyclines. Interestingly, doxycycline which resembles Minocycline hydrochloride structurally, has been associated with a short latency acute cholestatic or mixed hepatitis with immunoallergic features, but has not been associated definitively with the longer latency autoimmune hepatitis-like syndrome.
Minocycline hydrochloride as Treatment for Psychiatric and Neurological Conditions
Minocycline hydrochloride has anti-inflammatory, antioxidant, and anti-apoptotic properties that explain the renewed interest in its use as an adjunctive treatment for psychiatric and neurological conditions. Following the completion of several new clinical trials using Minocycline hydrochloride, we proposed an up-to-date systematic review and meta-analysis of the data available. The PICO (patient/population, intervention, comparison and outcomes) framework was used to search 5 databases aiming to identify randomized controlled trials that used Minocycline hydrochloride as an adjunctive treatment for psychiatric and neurological conditions. Search results, data extraction, and risk of bias were performed by two independent authors for each publication. Quantitative meta-analysis was performed using RevMan software. Literature search and review resulted in 32 studies being included in this review: 10 in schizophrenia, 3 studies in depression, and 7 in stroke, with the benefit of Minocycline hydrochloride being used in some of the core symptoms evaluated 2 in bipolar disorder and 2 in substance use, without demonstrating a benefit for using Minocycline hydrochloride 1 in obsessive-compulsive disorder, 2 in brain and spinal injuries, 2 in amyotrophic lateral sclerosis, 1 in Alzheimer's disease, 1 in multiple systems atrophy, and 1 in pain, with mixes results. For most of the conditions included in this review the data is still limited and difficult to interpret, warranting more well-designed and powered studies. On the other hand, the studies available for schizophrenia seem to suggest an overall benefit favoring the use of minocycline as an adjunctive treatment.[3]
References
[1] LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Minocycline.
[2] Davies MG, Kersey PJ. Acute hepatitis and exfoliative dermatitis associated with minocycline. BMJ 1989; 298: 1523-4.
[3] Panizzutti B, Skvarc D, Lin S, Croce S, Meehan A, Bortolasci CC, Marx W, Walker AJ, Hasebe K, Kavanagh BE, Morris MJ, Mohebbi M, Turner A, Gray L, Berk L, Walder K, Berk M, Dean OM. Minocycline as Treatment for Psychiatric and Neurological Conditions: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2023 Mar 9;24(6):5250.
- Related articles
- Related Qustion
Hydrocortisone acetate, a synthetic derivative of the natural steroid hormone hydrocortisone, is widely used in the pharmaceutical and healthcare industries.....
Apr 10,2025Chemical ReagentsCholine glycerophosphate (GPC) is a deacylated phosphatidylcholine derivative and is widely used as a dietary supplement.....
Mar 14,2025APIMinocycline hydrochloride
13614-98-7You may like
Minocycline hydrochloride manufacturers
- Minocycline hydrochloride
-

- $0.00 / 1kg
- 2025-04-10
- CAS:13614-98-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2500kg
- Minocycline hydrochloride
-

- $0.00 / 100g/Bag
- 2025-04-10
- CAS:13614-98-7
- Min. Order: 100g
- Purity: 890-950ug/mg; USP41
- Supply Ability: 400kg/month
- Minocycline hydrochloride
-

- $90.00 / 1kg
- 2025-04-08
- CAS:13614-98-7
- Min. Order: 1kg
- Purity: 99% Purity (What/sapp: +86 18145728414)
- Supply Ability: 1000 Tons/Month