GMP Human VEGF165 Protein
優(yōu)勢特色(Features)
1. Designed under ISO 9001:2015 and ISO 13485:2016
2. Manufactured and QC tested under a GMP compliance factory
3. Animal-Free materials
4. Beta-lactam materials free
5. Batch-to-batch consistency
6. Stringent quality control tests
表達(dá)區(qū)間及表達(dá)系統(tǒng)(Source)
GMP Human VEGF165 Protein (GMP-VE5H23) is expressed from human 293 cells (HEK293). It contains AA Ala 27 - Arg 191 (Accession # P15692-4).
Predicted N-terminus: Ala 27
蛋白結(jié)構(gòu)(Molecular Characterization)

This protein carries no "tag".
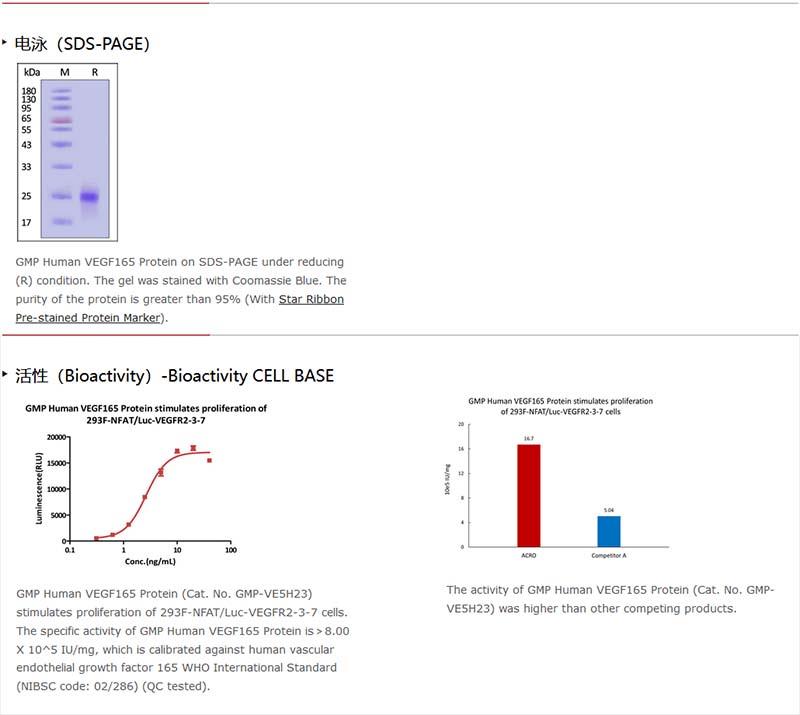
The protein has a calculated MW of 19.2 kDa. The protein migrates as 24 kDa±3 kDa when calibrated against Star Ribbon Pre-stained Protein Marker under reducing (R) condition (SDS-PAGE) due to glycosylation.
內(nèi)毒素(Endotoxin)
Less than 10 EU/mg by the LAL method.
宿主蛋白殘留(Host Cell Protein)
<0.5 ng/μg of protein tested by ELISA.
宿主核酸殘留(Host Cell DNA)
<0.02 ng/μg of protein tested by qPCR.
無菌(Sterility)
The sterility testing was performed by membrane filtration method described in CP<1101>, USP<71> and Eur. Ph. 2.6.1.
支原體(Mycoplasma)
Negative.
純度(Purity)
>95% as determined by SDS-PAGE.
制劑(Formulation)
Lyophilized from 0.22 μm filtered solution in PBS, pH7.4 with protectants.
Contact us for customized product form or formulation.
運(yùn)輸(Shipping)
This product is supplied and shipped with blue ice, please inquire the shipping cost.
存儲(chǔ)(Storage)
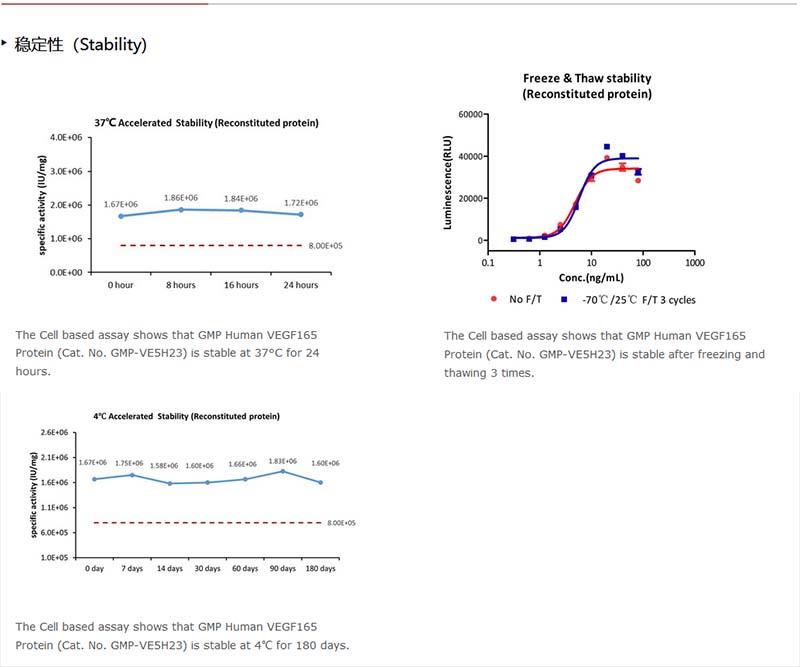
Upon receipt, store it immediately at -20°C or lower for long term storage.
Please avoid repeated freeze-thaw cycles.
This product is stable after storage at:
-20°C to -70°C for 5 years in lyophilized state;
-70°C for 12 months under sterile conditions after reconstitution.
ACRO GMP產(chǎn)品制造規(guī)范
ACROBiosystems GMP級產(chǎn)品是在質(zhì)量管理體系下生產(chǎn)的,并符合相關(guān)指南:
Ph. Eur General Chapter 5.2.12 Raw materials of biological origin for the production of cell-based and gene therapy medicinal products; USP <92> Growth Factors and Cytokines Used in Cell Therapy Manufacturing; USP <1043> Ancillary Materials for Cell, Gene, and Tissue-Engineered Products; ISO/TS 20399-1:2018, Biotechnology - Ancillary Materials Present During the Production of Cellular Therapeutic Products.
ACROBiosystems質(zhì)量管理體系內(nèi)容:
1. 根據(jù)ISO 9001:2015和ISO 13485:2016進(jìn)行設(shè)計(jì)���,在GMP工廠進(jìn)行制造和QC檢測
2. 無動(dòng)物成分
3. QA從批準(zhǔn)的供應(yīng)商處采購的材料
4. ISO 5潔凈室和自動(dòng)灌裝設(shè)備
5. 人員合格
6. 質(zhì)量保證審核和批準(zhǔn)質(zhì)量相關(guān)文件
7. 全批量生產(chǎn)和控制記錄
8. 設(shè)備維護(hù)和校準(zhǔn)
9. 分析程序的驗(yàn)證
10. 進(jìn)行的穩(wěn)定性研究
11. 全面的法規(guī)支持文件
ACROBiosystems對我們的GMP級產(chǎn)品提供嚴(yán)格的質(zhì)量控制測試(經(jīng)過充分驗(yàn)證的設(shè)備��、工藝和測試方法)��,以確保它們在純度���、安全性、活性和批間穩(wěn)定性方面符合嚴(yán)格的標(biāo)準(zhǔn)�����,每個(gè)批量QC批次主要包含以下具體信息:
1. SDS-PAGE
2. 蛋白質(zhì)含量
3. 內(nèi)毒素水平
4. 殘留宿主細(xì)胞DNA含量
5. 殘留宿主細(xì)胞蛋白質(zhì)含量
6. 生物活性分析
7. 微生物檢測
8. 支原體檢測
9. 體外病毒測定
10. 殘留水分
11. 批次間一致性
ACRO產(chǎn)品聲明
ACROBiosystems GMP級產(chǎn)品專為研究���、生產(chǎn)或離體使用而設(shè)計(jì)�。注意:不可直接供人體使用。

背景(Background)
VEGF165是VEGF-A最豐富的剪接變體���。VEGF165由許多細(xì)胞產(chǎn)生�,包括內(nèi)皮細(xì)胞����、巨噬細(xì)胞和T細(xì)胞。VEGF165參與血管生成�、血管內(nèi)皮細(xì)胞存活、生長�����、遷移和血管通透性�。VEGF基因表達(dá)是由缺氧、炎性細(xì)胞因子和癌基因誘導(dǎo)的�。VEGF165與硫酸乙酰肝素結(jié)合,并保留在細(xì)胞表面和細(xì)胞外基質(zhì)中���。VEGF165可與受體酪氨酸激酶VEGFR1和VEGFR2結(jié)合���。VEGF165是唯一一種與共受體NRP-1和NRP-2結(jié)合的剪接變異體,其功能是增強(qiáng)VEGFR2信號傳導(dǎo)。VEGF165與VEGFR1和VEGFR2的結(jié)合導(dǎo)致PI3K/AKT�、p38 MAPK、FAK和帕羅西汀的激活����。VEGF在許多癌癥的腫瘤血管生成中起著關(guān)鍵作用。
關(guān)鍵字: GMP VEGF165;VEGF165;VEGF165蛋白;ACRO;百普賽斯;
百普賽斯集團(tuán)ACROBiosystems Group(股票代碼:301080)是成立于2010年的跨國生物科技公司����,是為全球生物醫(yī)藥、健康產(chǎn)業(yè)領(lǐng)域提供關(guān)鍵生物試劑產(chǎn)品及解決方案的行業(yè)平臺型基石企業(yè)��。2021年在創(chuàng)業(yè)板上市�。百普賽斯集團(tuán)業(yè)務(wù)遍布全球�����,橫跨亞洲�����、北美洲���、歐洲����,在中國、美國����、瑞士等12個(gè)城市設(shè)有辦公室、研發(fā)中心及生產(chǎn)基地�����。目前累計(jì)服務(wù)客戶超6000家����,與全球Top 20醫(yī)藥企業(yè)均建立了長期、穩(wěn)定的合作伙伴關(guān)系�。集團(tuán)旗下?lián)碛衅放艫CROBiosystems百普賽斯、bioSeedin柏思薈��、Condense Capital墾拓資本和ACRODiagnostics百斯醫(yī)學(xué)等�。