| 77% |
With ammonia; tetra-(n-butyl)ammonium iodide; N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; methanol; chloroform; |
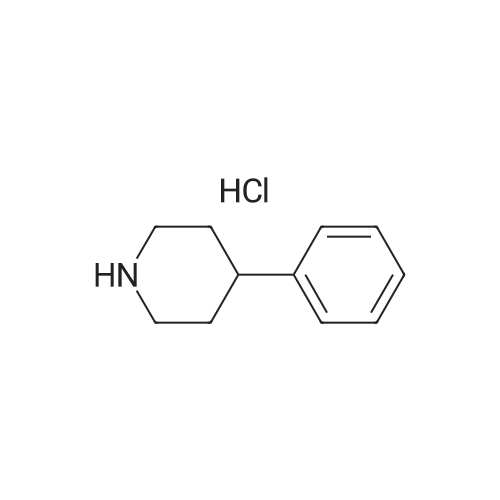
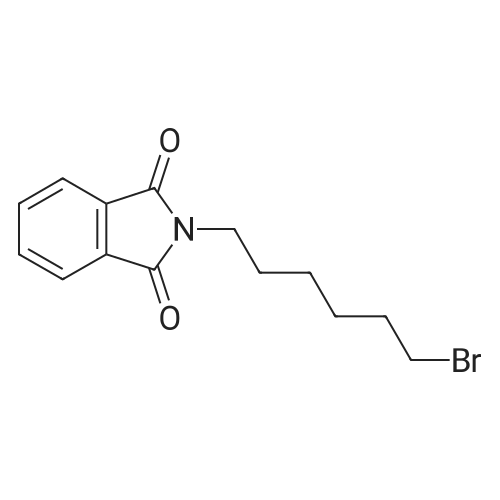
2-[6-(4-phenyl-1-piperidinyl)hexyl]-1H-isoindole-1,3(2H)-dione: To the 500 ml RB-flask was added <strong>[10272-49-8]4-phenylpiperidine hydrochloride</strong> (5 g, 25 mmol), N-(6-bromohexyl)phthalimide (15.5 g, 50 mmol), N,N-diisopropylethylamine (21.8 ml, 125 mmol), tetrabutylammonium iodide (0.2 g), and dioxane (250 ml) at room temperature. The reaction mixture was stirred at 100 oC for 72 h. The solvent was removed in vacuo and the crude product was purified by flash chromatography (98:2=Chloroform: 2N ammonia in methanol) to afford 7.67 g of the desired product (77% yield): 1H NMR (400 MHz, CDCl3) delta 7.78-7.79 (m, 2H), 7.74-7.65 (m, 2H), 7.32-7.14 (m, 5H), 3.69 (t, 2H, J=7.35 Hz), 3.06 (d, 2H, J=11.0 Hz), 2.49 (quintet, 1H, J=7.6 Hz), 2.36 (t, 2H, J=7.6 Hz), 2.02 (t, 2H, J=12.5 Hz), 1.82 (br s, 4H), 1.69 (t, 2H, J=6.3 Hz), 1.54 (br s, 2H), 1.37 (br s, 4H); ESMS m/e: 391.3 (M+H) +; Anal. Calc. for C25H30N2O2+0.2H2O: C, 76.19; H, 7.77; N, 7.11. Found: C, 76.14; H, 7.38; N, 7.13. |
| 77% |
With ammonia; tetra-(n-butyl)ammonium iodide; N-ethyl-N,N-diisopropylamine; In 1,4-dioxane; methanol; chloroform; |
2-[6-(4-PHENYL-1-PIPERIDINYL)HEXYL]-1H-ISOINDOLE-1,3(2H)-DIONE: To the 500 ml RB-flask was added <strong>[10272-49-8]4-phenylpiperidine hydrochloride</strong> (5 g, 25 mmol), N-(6-bromohexyl)phthalimide (15.5 g, 50 mmol), N,N-diisopropylethylamine (21.8 ml, 125 mmol), tetrabutylammonium iodide (0.2 g), and dioxane (250 ml) at room temperature. The reaction mixture was stirred at 100 C. for 72 h. The solvent was removed in vacuo and the crude product was purified by flash chromatography (98:2=Chloroform: 2N ammonia in methanol) to afford 7.67 g of the desired product (77% yield): 1H NMR (400 MHz, CDCl3) delta 7.78-7.79 (m, 2H), 7.74-7.65 (m, 2H), 7.32-7.14 (m, 5H), 3.69 (t, 2H, J=7.35 Hz), 3.06 (d, 2H, J=11.0 Hz), 2.49 (quintet, 1H, J=7.6 Hz), 2.36 (t, 2H, J=7.6 Hz), 2.02 (t, 2H, J=12.5 Hz), 1.82 (br s, 4H), 1.69 (t, 2H, J=6.3 Hz), 1.54 (br s, 2H), 1.37 (br s, 4H); ESMS m/e: 391.3 (M+H)+; Anal. Calc. for C25H30N2O2+0.2H2O: C, 76.19; H, 7.77; N, 7.11. Found: C, 76.14; H, 7.38; N, 7.13. |
| 77% |
With N-ethyl-N,N-diisopropylamine;tetra-(n-butyl)ammonium iodide; In 1,4-dioxane; at 100℃; for 72h; |
To the 500 ml RB-flask was added <strong>[10272-49-8]4-phenylpiperidine hydrochloride</strong> (5 g, 25 mmol), N-(6-bromohexyl)phthalimide (15.5 g, 50 mmol), N,N-diisopropylethylamine (21.8 ml, 125 mmol), tetrabutylammonium iodide (0.2 g), and dioxane (250 ml) at room temperature. The reaction mixture was stirred at 100 C. for 72 h. The solvent was removed in vacuo and the crude product was purified by flash chromatography (98:2=Chloroform:2N ammonia in methanol) to afford 7.67 g of the desired product (77% yield): 1H NMR (400 MHz, CDCl3) delta 7.78-7.79 (m, 2H), 7.74-7.65 (m, 2H), 7.32-7.14 (m, 5H), 3.69 (t, 2H, J=7.35 Hz), 3.06 (d, 2H, J=11.0 Hz), 2.49 (quintet, 1H, J=7.6 Hz), 2.36 (t, 2H, J=7.6 Hz), 2.02 (t, 2H, J=12.5 Hz), 1.82 (br s, 4H), 1.69 (t, 2H, J=6.3 Hz), 1.54 (br s, 2H), 1.37 (br s, 4H); ESMS m/e: 391.3 (M+H)+; Anal. Calc. for C25H30N2O2+0.2H2O: C, 76.19; H, 7.77; N, 7.11. Found: C, 76.14; H, 7.38; N, 7.13. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping