| 33% |
With benzotriazol-1-ol; dicyclohexyl-carbodiimide; In tetrahydrofuran; at 20℃;Inert atmosphere; |
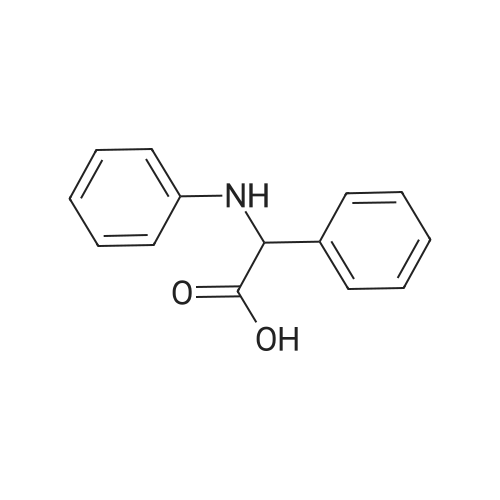
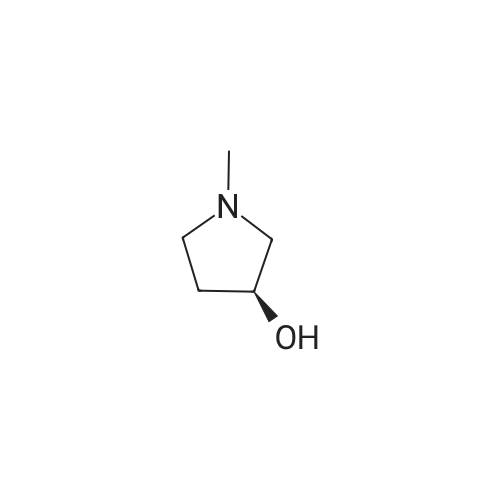
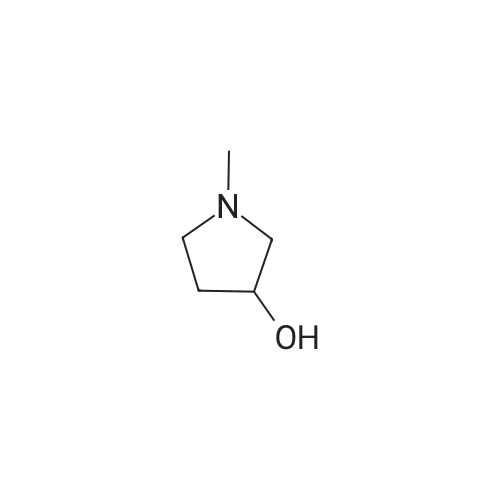
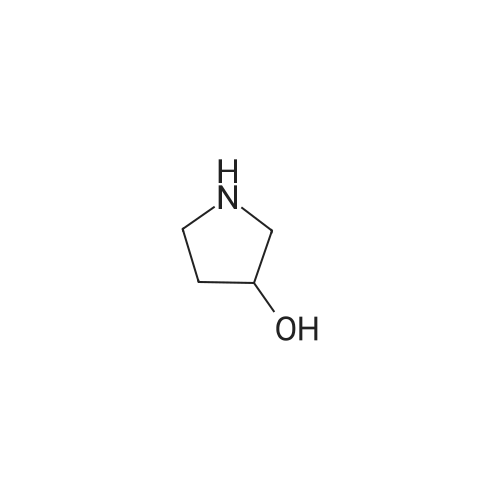
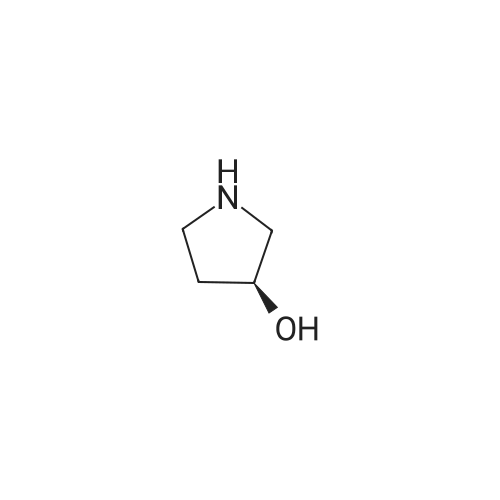
A mixture of <strong>[3684-12-6]2-phenyl-2-(phenylamino)acetic acid</strong> (II) (200 mg, 0.88 mmol), DCC (218 mg, 1.05 mmol), HOBt (142 mg, 1.05 mmol) and (R)- I- methylpyrrolidin-3-ol (289 uL, 2.64 mmol) in dry THF (10 mL) is stirred at room temperature overnight under nitrogen flowstream (LC-MS monitoring: complete conversion). The solvent is evaporated and the residue is taken up with aq. HCl (pH about 2) and washed with DCM. The aqueous phase is basified with NaHCO3 and extracted with DCM (three times). The organic layers are combined, dried over Na2SO4, filtered and evaporated to dryness. The resulting crude is first purified by flash chromatography (DCM to DCM/MeOH=95/5) and then by preparative LC-MS. The purified compound is partitioned between sat. NaHCO3 and DCM, the organic phase is dried over Na2SO4, filtered and evaporated under vacuum to give 90.8 mg of the title compound as brown oil (33% yield, mixture of diastereoisomers).1H NMR (300 MHz, CHLOROFORM-d) ppmDiastereoisomer 1 of C 14: 7.46 - 7.57 (m, 2 H), 7.29 - 7.45 (m, 3 H), 7.08 - 7.21 (m, 2 H), 6.67 - 6.81 (m, 1 H), 6.50 - 6.67 (m, 2 H), 5.20 - 5.37 (m, 1 H), 5.12 (d, 1 H), 4.84 - 5.05 (m, 1 H), 2.46 - 3.04 (m, 4 H), 2.44 (s, 3 H), 2.10 - 2.26 (m, 1 H), 1.63 - 1.82 (m, 1 H). Diastereoisomer 2 of C14: 7.46 - 7.57 (m, 2 H), 7.29 - 7.45 (m, 3 H), 7.08 - 7.21 (m, 2 H), 6.67 - 6.81 (m, 1 H), 6.50 - 6.67 (m, 2 H), 5.20 - 5.37 (m, 1 H), 5.12 (d, 1 H), 4.84 - 5.05 (m, 1 H), 2.46 - 3.04 (m, 4 H), 2.33 (s, 3 H), 2.26 - 2.40 (m, 1 H), 1.86 - 2.05 (m, 1 H);LC-MS (ESI POS): 31 1.3 (MH+). |
| 33% |
With benzotriazol-1-ol; dicyclohexyl-carbodiimide; In tetrahydrofuran; at 20℃; for 40h;Inert atmosphere; |
A mixture of <strong>[3684-12-6]2-phenyl-2-(phenylamino)acetic acid</strong> (II) (200 mg, 0.88 mmol), DCC (218 mg, 1.05 mmol), HOBt (142 mg, 1.05 mmol) and (R)-l- methylpyrrolidin-3-ol (289 uL, 2.64 mmol) in dry THF (10 mL) was stirred at room temperature overnight under nitrogen flowstream (LC-MS monitoring: complete conversion). The solvent was evaporated and the residue was taken up with aq. HCl (pH about 2) and washed with DCM. The aqueous phase was basified with NaHCO3 and extracted with DCM (three times). The organic layers were combined, dried over Na2SO4, filtered and evaporated to dryness. The resulting crude was first purified by flash chromatography (DCM to DCM/MeOH=95/5) and then by preparative LC-MS. The purified compound was partitioned between sat. NaHCO and DCM, the organic phase was dried over Na2SO4, filtered and evaporated under vacuum to give 90.8 mg of the title compound (33% yield, mixture of diastereomers).1H NMR (300 MHz, CHLOROFORM-d) ppmDiastereomer 1 of 133: 7.46 - 7.57 (m, 2 H), 7.29 - 7.45 (m, 3 H), 7.08 - 7.21 (m, 2 H), 6.67 - 6.81 (m, 1 H), 6.50 - 6.67 (m, 2 H), 5.20 - 5.37 (m, 1 H), 5.12 (d, 1 H), 4.84 - 5.05 (m, 1 H), 2.46 - 3.04 (m, 4 H), 2.44 (s, 3 H), 2.10 - 2.26 (m, 1 H), 1.63 - 1.82 (m, 1 H).Diastereomer 2 of 133: 7.46 - 7.57 (m, 2 H), 7.29 - 7.45 (m, 3 H), 7.08 - 7.21 (m, 2 H), 6.67 - 6.81 (m, 1 H), 6.50 - 6.67 (m, 2 H), 5.20 - 5.37 (m, 1 H), 5.12 (d, 1 H), 4.84 - 5.05 (m, 1 H), 2.46 - 3.04 (m, 4 H), 2.33 (s, 3 H), 2.26 - 2.40 (m, 1 H), 1.86 - 2.05 (m, 1 H);LC-MS (ESI POS): 31 1.3 (MH+). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping