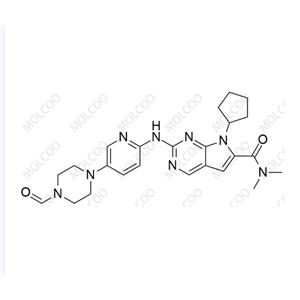
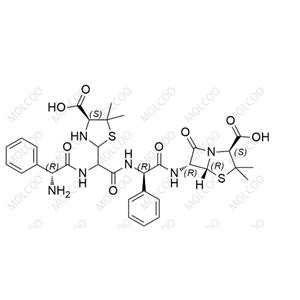
Ribociclib Impurity 14 NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 100000 |
| Update Time: | 2025-01-22 |
Product Details
| Product Name: Ribociclib Impurity 14 | CAS No.: 2460732-08-3 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 100000 | Release date: 2025/01/22 |
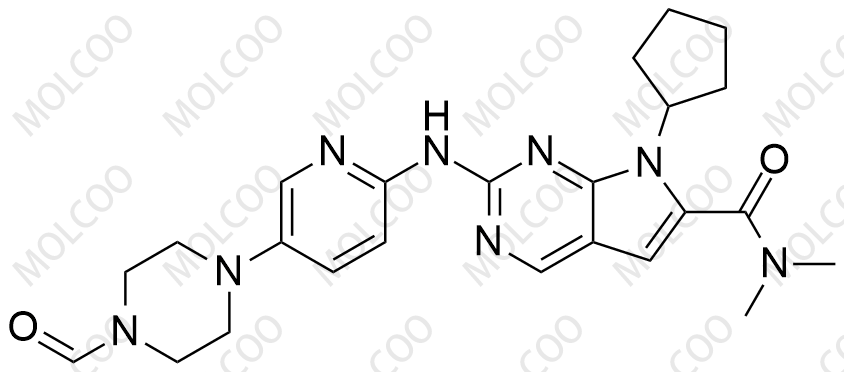
Ribociclib Impurity Reference Standards
Ribociclib impurity reference standards play a vital role in drug research and development, quality control, and pharmaceutical testing. Ribociclib, a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor, is primarily used for the treatment of patients with HR-positive, HER2-negative breast cancer. To ensure the quality and safety of Ribociclib products, precise identification and quantitative analysis of its impurities are essential steps.
Our Ribociclib impurity reference standards cover a variety of key impurities, including but not limited to Ribociclib N-Oxide and N-desmethyl Ribociclib. These impurity reference standards have been meticulously prepared and undergone rigorous quality control to ensure their purity, chemical structure, and stability comply with international and industry standards.
By utilizing Ribociclib impurity reference standards, drug researchers and quality control personnel can more accurately identify and quantitatively analyze impurity components in pharmaceutical products, thereby effectively monitoring the production process and ensuring the quality and safety of the final products.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/1g |
VIP2Y
|
shandong perfect biotechnology co.ltd
|
2023-08-02 | |
| $0.00/1g |
VIP1Y
|
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
|
2024-08-09 | |
| $36.00/2mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake




 China
China