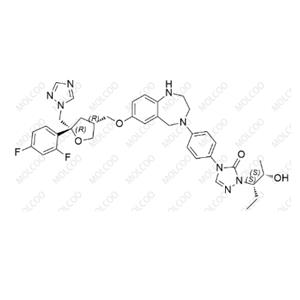
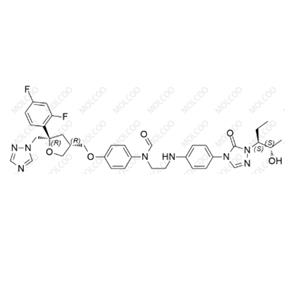
Posaconazole Impurity NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 100000 |
| Update Time: | 2025-01-17 |
Product Details
| Product Name: Posaconazole Impurity | CAS No.: 1388148-30-8 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 100000 | Release date: 2025/01/17 |
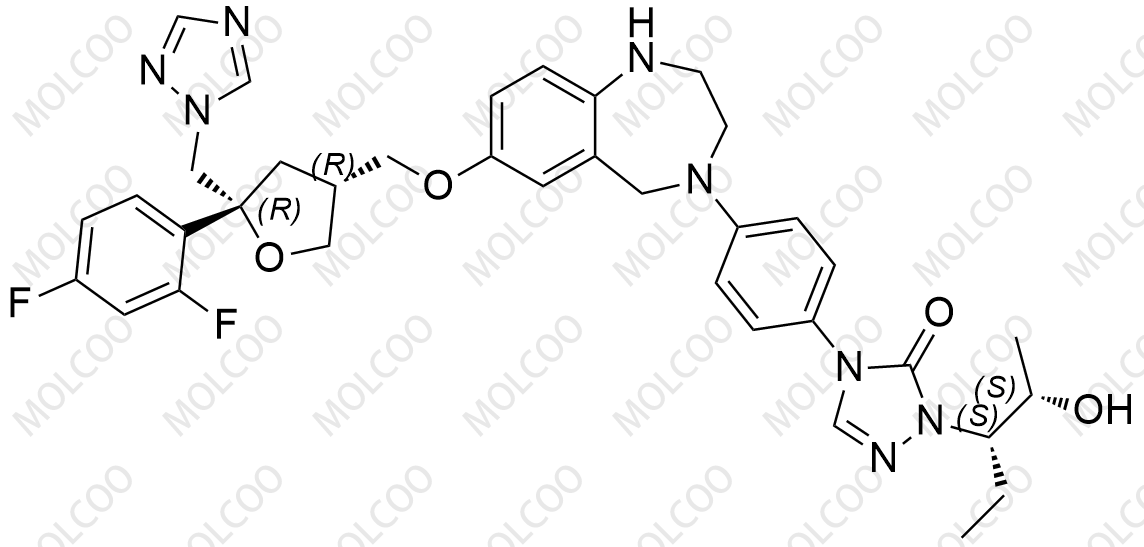
Posaconazole Impurity Reference Standards
Posaconazole is an important antifungal drug. To ensure its quality and purity, we need to conduct strict comparison and analysis of impurities in Posaconazole. Here is the information on Posaconazole impurity reference standards we provide:
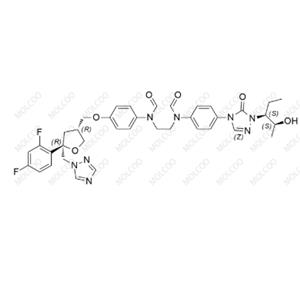
Posaconazole Impurity 01
CAS Number: 159811-30-0
Molecular Formula: C21H21F2N3O4S
Molecular Weight: 449.47
Posaconazole Impurity 02
CAS Number: Not Available
Molecular Formula: C21H21F2N3O4S
Molecular Weight: 449.47
(Note: Due to space limitations, only two impurities are listed here as examples. The actual product may contain more types of impurities.)
These impurity reference standards can be used for quality control, drug development, and impurity detection and analysis in the production process of Posaconazole. Through precise impurity comparison, we can ensure the quality and purity of Posaconazole products, thereby guaranteeing the safety and efficacy of medication for patients.
We provide high-quality Posaconazole impurity reference standards, produced and tested strictly according to relevant standards to ensure their accuracy and reliability. If you have any needs, please contact us, and we will be happy to provide you with quality products and services

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2024-12-31 | |
| $17742.00/1KG |
VIP4Y
|
Baoji Guokang Bio-Technology Co., Ltd.
|
2021-06-02 | |
| $0.00/10mg |
VIP1Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2024-07-11 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake





 China
China