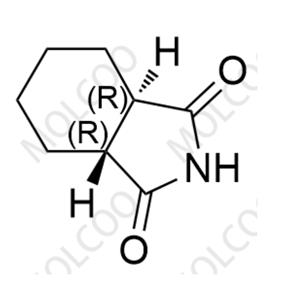
Product Details
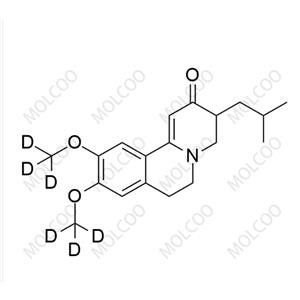
| Product Name: Perospirone Impurity | CAS No.: 1002359-81-0 |
| Min. Order: 10mg | Purity: 98 |
| Supply Ability: 100000000 | Release date: 2025/02/05 |
We understand that impurities are not just about the purity of a drug; they directly relate to patient safety and efficacy. Therefore, we are dedicated to the research and control of Perospirone impurities, utilizing advanced detection technologies to precisely identify and remove every trace of impurities that may compromise drug quality.
Our testing and analysis laboratory is equipped with a variety of foreign imported testing equipment such as LC-MS, liquid phase, TGA, infrared, etc., to escort research and development. At the same time, it also provides customers with faster, more true and accurate product quality confirmation. For impurities with different properties, MOLCOO provides accurate impurity adaptation environment (-80°C, -20°C, 2-8°C), and uses different adapted reagent bottles to ensure the stable storage of impurity controls in various environments. Worry-free after-sales! If there is a problem with the quality of the sample, we promise to return it quickly and refund unconditionally!


Company Profile Introduction
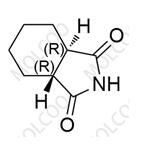
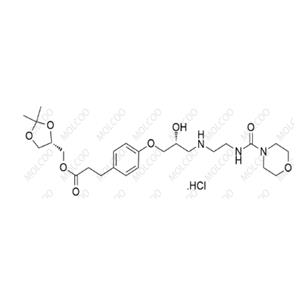
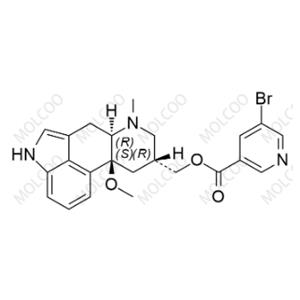
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $37.00/5mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $1980.00/5mg |
VIP3Y
|
TargetMol Chemicals Inc.
|
2024-10-28 | |
| $0.00/5mg |
VIP1Y
|
TargetMol Chemicals Inc.
|
2024-10-28 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake





 China
China