
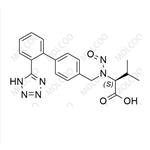
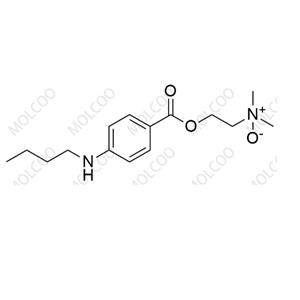
N-Nitroso LCZ696 NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 100000 |
| Update Time: | 2025-01-21 |
Product Details
| Product Name: N-Nitroso LCZ696 | CAS No.: 2254485-68-0 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 100000 | Release date: 2025/01/21 |
The Sacubitril Valsartan Impurity Reference Standard is an essential reagent in drug research and development, as well as quality control. It is used to verify and detect the impurity content in Sacubitril Valsartan drugs, ensuring the quality and safety of the medication. Our Sacubitril Valsartan Impurity Reference Standard is carefully prepared and undergoes rigorous quality control, ensuring high purity and stability.
This reference standard is suitable for various analytical techniques, such as High-Performance Liquid Chromatography (HPLC), Nuclear Magnetic Resonance (NMR), and Mass Spectrometry (MS), to meet the needs of different laboratories. By using our Sacubitril Valsartan Impurity Reference Standard, you can accurately identify and quantify impurities in the drug, providing reliable data support for drug research and development, production, and quality control.
Please note that this product is intended for research and industrial production use only and should not be used for human treatment or diagnosis. Store the product according to the instructions on the packaging to ensure its stability and effectiveness.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/1g |
VIP1Y
|
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
|
2024-09-19 | |
| $0.00/1kg |
VIP1Y
|
Wuhan Circle Star Chem-medical Technology Co.,Ltd
|
2025-01-14 | |
| $110.00/10mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-10-28 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake




 China
China