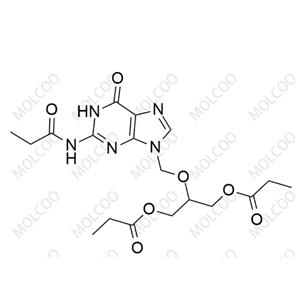
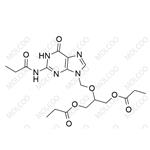
Ganciclovir EP Impurity J NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 100000 |
| Update Time: | 2025-01-18 |
Product Details
| Product Name: Ganciclovir EP Impurity J | CAS No.: 177216-32-9 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 100000 | Release date: 2025/01/18 |
Ganciclovir Impurity Reference Standards
Ganciclovir impurity reference standards are indispensable tools in the fields of drug research and development, quality control, and pharmaceutical testing. Ganciclovir, as a highly effective and broad-spectrum antiviral drug, is widely used in clinical treatment. To ensure the purity and safety of Ganciclovir products, accurate identification and quantitative analysis of Ganciclovir and its impurities are crucial.
Our Ganciclovir impurity reference standards cover a variety of key impurities, including but not limited to Ganciclovir EP Impurity A, Ganciclovir Impurity C, Ganciclovir Impurity C-D5, and Ganciclovir Impurity H. These impurity reference standards have undergone rigorous quality control and purity testing to ensure they meet international and industry standards.
With Ganciclovir impurity reference standards, you can:
Accurately Identify Impurities: By comparing with impurity reference standards, you can quickly and accurately identify impurity components in pharmaceutical products.
Quantitatively Analyze Impurity Content: Utilize impurity reference standards for quantitative analysis to ensure that the impurity content in pharmaceutical products meets the specified standards.
Improve Pharmaceutical Quality Control: During pharmaceutical production and research and development, impurity reference standards can serve as important quality control tools to help improve the overall quality of pharmaceutical products.
We are committed to providing high-quality and high-purity Ganciclovir impurity reference standards to meet our customers' needs in drug research and development, quality control, and pharmaceutical testing.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $457.00/1mg |
VIP3Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $0.00/5mg |
VIP1Y
|
Guangzhou Tosun Pharmaceutical Ltd
|
2025-01-15 | |
| $10.00/1kg |
VIP2Y
|
Henan Fengda Chemical Co., Ltd
|
2024-04-29 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake




 China
China