FEXUPRAZAN / ABEPRAZAN
| Price | Get Latest Price | |
| Package | 1kg | 25kg |
| Min. Order: | 1kg |
| Supply Ability: | 900kg |
| Update Time: | 2025-03-03 |
Product Details
| Product Name: FEXUPRAZAN / ABEPRAZAN | CAS No.: 1902954-60-2 |
| Min. Order: 1kg | Purity: 0.99 |
| Supply Ability: 900kg | Release date: 2025/03/03 |
? ?Fexuprazan (CAS 1902954-60-2) | Next-Gen Potassium-Competitive Acid Blocker (P-CAB) for Gastrointestinal Disorders? ?
?? ?Product Overview?
?Fexuprazan? (CAS 1902954-60-2), also known as ?Abeprazan?, is a ?potassium-competitive acid blocker (P-CAB)? with the molecular formula ?C??H??F?N?O?S? and molecular weight ?410.41?. This high-purity white to off-white crystalline powder (≥95% HPLC) is designed for ?acid-related gastrointestinal therapies?, including ?erosive esophagitis? and ?peptic ulcer disease?. Developed by Daewoong Pharmaceuticals, it offers rapid and sustained acid suppression by inhibiting H+/K+-ATPase, outperforming traditional proton pump inhibitors (PPIs) in symptom relief and mucosal healing.
?Key Functions?:
Accelerates ?erosive esophagitis healing? and reduces recurrence risk.
Enhances ?Helicobacter pylori eradication? in combination therapies.
Acts as a ?pharmaceutical intermediate? for advanced P-CAB formulations.
?Target Industries?: Pharmaceutical manufacturing, clinical research, biotechnology.
? ?Key Advantages?
| ≥95% HPLC-certified | Acid suppression within 30 minutes | Combines ulcer healing and H. pylori eradication |
| Batch-specific COA | Superior to PPIs in nocturnal symptom relief | Stable at -20°C for long-term storage |
?? ?Applications?
?Erosive Esophagitis?: Oral formulations for mucosal repair and symptom control.
?Peptic Ulcers?: Reduces gastric and duodenal ulcer recurrence rates.
?H. pylori Therapy?: Synergistic use with antibiotics for higher eradication success.
?Drug Development?: Intermediate for novel P-CAB-based therapies.
?? ?Quality Assurance?
?Testing Standards?: Complies with ?USP/EP? pharmacopeial guidelines.
?Analytical Methods?: Validated via ?HPLC, NMR?, and ?mass spectrometry?.
?Packaging?: Customizable batches (10mg–1kg) with moisture-proof, light-protected containers.
?? ?Market Insights?
The global gastroesophageal reflux disease (GERD) therapeutics market is projected to grow at ?6.5% CAGR (2025–2030)?, driven by rising demand for non-PPI therapies. Asia-Pacific leads P-CAB API production, with South Korea and China dominating innovation and cost-efficient manufacturing.

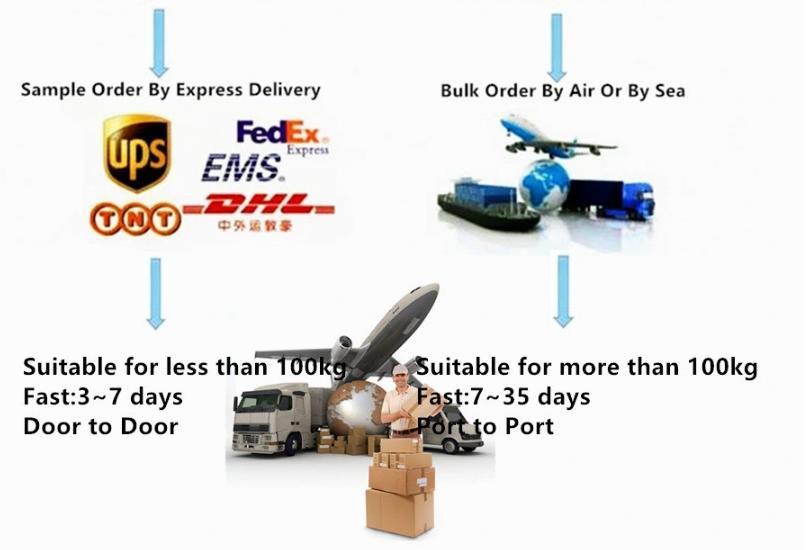

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $94.00/1mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-01 | |
| $94.00/1mg |
VIP3Y
|
TargetMol Chemicals Inc.
|
2024-11-01 | |
| $0.00/1kg |
VIP1Y
|
Hebei Junhua Import and Export Co., LTD
|
2025-02-19 | |
| $1980.00/50mg |
VIP3Y
|
TargetMol Chemicals Inc.
|
2024-10-28 |
- Since: 2018-06-08
- Address: Biolake.858 high-tech avenue,Wuhan
+86-+undefined-+86 13343427080
sales@biocarchem.com









 China
China