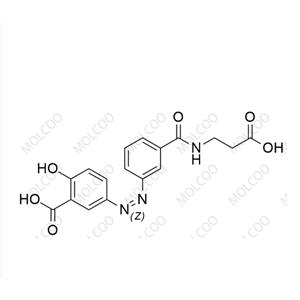
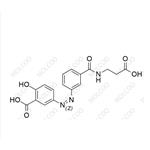
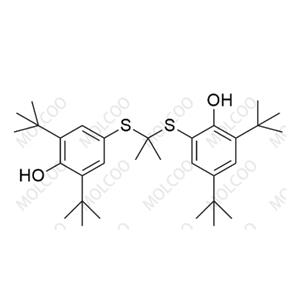
Balsalazide USP Related Compound B NEW
| Price | Get Latest Price | ||
| Package | 10mg | 30mg | 100mg |
| Min. Order: | 10mg |
| Supply Ability: | 10000 |
| Update Time: | 2025-03-08 |
Product Details
| Product Name: Balsalazide USP Related Compound B | CAS No.: 1798395-96-6 |
| Min. Order: 10mg | Purity: 95%+ |
| Supply Ability: 10000 | Release date: 2025/03/08 |
Product Overview
Balsalazide impurity reference standards are essential standard substances in the scientific research field, primarily used for identification, inspection, content determination, and analysis of impurities and related substances. These reference standards are strictly produced in accordance with national pharmaceutical standards, ensuring their accuracy and reliability in scientific research applications.
Product Features
High Purity: Balsalazide impurity reference standards have extremely high purity, ensuring the accuracy and reproducibility of research results.
Multiple Specifications: Various packaging specifications are available to meet the needs of different research projects.
Strict Quality Control: Each batch of products undergoes rigorous quality control system testing to ensure stable and reliable product quality.
Professional Use: For research use only, not for medical diagnosis or other non-research purposes.

Company Profile Introduction
You may like
Recommended supplier
| Product name | Price | Suppliers | Update time | |
|---|---|---|---|---|
| $0.00/10mg |
VIP1Y
|
ShenZhen H&D Pharmaceutical Technology Co., LTD
|
2025-02-06 | |
| $29.00/50mg |
VIP5Y
|
TargetMol Chemicals Inc.
|
2024-11-19 | |
| $75.00/1mg |
VIP3Y
|
TargetMol Chemicals Inc.
|
2024-11-19 |
- Since: 2022-11-29
- Address: Room 005-01, 15th Floor, Building D2, Phase III, Software New Town, No. 8 Huacheng Avenue, East Lake




 China
China