| Company Name: |
Suzhou Meishi Biotechnology Co., Ltd.
|
| Tel: |
1173954148q |
| Email: |
meishipharma@126.com |
| Products Intro: |
Product Name:132562-26-6
CAS:132562-26-6
Purity:99% HPLC Package:1G;5G;10G;100G;1KG;
|
| Company Name: |
Shanghai Yifei Biotechnology Co. , Ltd.
|
| Tel: |
021-65675885 18964387627 |
| Email: |
customer_service@efebio.com |
| Products Intro: |
Product Name:Aprikalim
CAS:132562-26-6
Purity:99.00% Package:1mg;5mg;10mg
|
| Company Name: |
TargetMol Chemicals Inc.
|
| Tel: |
15002134094 |
| Email: |
marketing@targetmol.cn |
| Products Intro: |
Product Name:Aprikalim
CAS:132562-26-6
Purity:99.38% Package:1mg/RMB 1980;5mg/RMB 4920;10mg/RMB 7130
|
(R)-N-Methyl-2-(3-pyridinyl)-3,4,5,6-tetrahydro-2H-thiopyran-2-carbothioamide 1-oxide manufacturers
- Aprikalim
-

- $293.00 / 1mg
-
2025-06-24
- CAS:132562-26-6
- Min. Order:
- Purity: 99.90%
- Supply Ability: 10g
|
| | (R)-N-Methyl-2-(3-pyridinyl)-3,4,5,6-tetrahydro-2H-thiopyran-2-carbothioamide 1-oxide Basic information |
| Product Name: | (R)-N-Methyl-2-(3-pyridinyl)-3,4,5,6-tetrahydro-2H-thiopyran-2-carbothioamide 1-oxide | | Synonyms: | (R)-2α-(Methylthiocarbamoyl)-1-oxylato-2-(3-pyridyl)hexahydrothiopyrylium;(R)-N-Methyl-2-(3-pyridinyl)-3,4,5,6-tetrahydro-2H-thiopyran-2-carbothioamide 1-oxide;RP-52891;Unii-374bh4kvrg;2H-Thiopyran-2-carbothioamide, tetrahydro-N-methyl-2-(3-pyridinyl)-, 1-oxide, (1R,2R)- | | CAS: | 132562-26-6 | | MF: | C12H16N2OS2 | | MW: | 268.4 | | EINECS: | | | Product Categories: | | | Mol File: | 132562-26-6.mol | 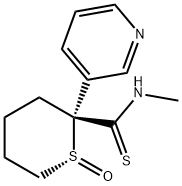 |
| | (R)-N-Methyl-2-(3-pyridinyl)-3,4,5,6-tetrahydro-2H-thiopyran-2-carbothioamide 1-oxide Chemical Properties |
| Boiling point | 493.5±55.0 °C(Predicted) | | density | 1.32±0.1 g/cm3(Predicted) | | pka | 11.90±0.20(Predicted) |
| | (R)-N-Methyl-2-(3-pyridinyl)-3,4,5,6-tetrahydro-2H-thiopyran-2-carbothioamide 1-oxide Usage And Synthesis |
| Uses | Aprikalim (RP 52891), a potassium channel opener (KCO), activates ATP-sensitive K+ (KATP) channels in guinea pig ventricular myocytes. Using patch-clamp techniques, it was found that aprikalim enhances KATP channel activity more effectively in Mg-NDP solution compared to standard solutions. In Mg-NDP solution, aprikalim reduced the sensitivity of KATP channels to ATP, increasing the concentration of ATP required to inhibit channel activity by half (K1) from 56 μM to 180 μM. However, this effect diminished over time. Aprikalim's ability to activate KATP channels in Mg-NDP solution suggests potential therapeutic implications in modulating cardiac excitability and may relate to changes in channel protein enzymatic activity under experimental conditions[1]. | | References | [1] D Thuringer, et al. Time-dependent fading of the activation of KATP channels, induced by aprikalim and nucleotides, in excised membrane patches from cardiac myocytes. Br J Pharmacol. 1995 May;115(1):117-27. DOI:10.1111/j.1476-5381.1995.tb16328.x |
| | (R)-N-Methyl-2-(3-pyridinyl)-3,4,5,6-tetrahydro-2H-thiopyran-2-carbothioamide 1-oxide Preparation Products And Raw materials |
|