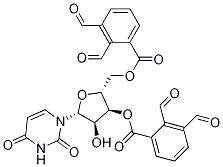
3',5'-Di-O-benzoyl Fialuridine synthesis
- Product Name:3',5'-Di-O-benzoyl Fialuridine
- CAS Number:97614-45-4
- Molecular formula:C23H18FIN2O7
- Molecular Weight:580.3
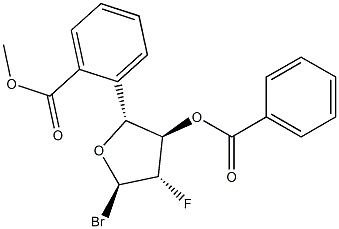
97614-44-3
63 suppliers
$45.00/100mg
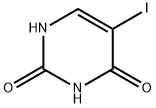
696-07-1
300 suppliers
$20.00/5g

97614-45-4
28 suppliers
$160.00/25mg
Yield:97614-45-4 54.3%
Reaction Conditions:
Stage #1: 5-iodouracilwith N,O-bis-(trimethylsilyl)-acetamide in 1,2-dichloro-ethane at 20; for 5 h;
Stage #2: 2-deoxy-2-fluoro-3,5-di-O-benzoyl-α-D-arabinofuranosyl bromide in 1,2-dichloro-ethane; for 22 h;Reflux;
Steps:
5.3.1
7.08 g (29.75 mmol) 5-iodouracil are suspended in 105 ml 1.2-dichloroethane. 13.32 g (65.47 mmol) N,O-bis-trimethylsilylacetamide (BSA) are added thereto and agitated for 5 h at room temperature. A clear, colourless solution is produced. 11.40 g (26.9 mmol) 3,5-di-O-benzoyl-2-deoxy-2-fluoro-α-D-arabinosyl-bromide are added thereto. This is heated for 22 h at reflux. The conversion is thereafter complete (DC-control with chloroform/methanol 100:1). The batch is diluted with 450 ml acetic ester and treated with 400 ml phosphate buffer (pH=7). The organic phase is separated and the aqueous one extracted with 3×150 ml acetic ester. After drying the combined organic phases with magnesium sulphate, filtering-off of the drying agent and distilling-off of the solvent, recrystallisation takes place from isopropyl alcohol. 8.47 g (54.3%) of a colourless product is obtained.
References:
US2010/227834,2010,A1 Location in patent:Page/Page column 18
38953-72-9
3 suppliers
inquiry
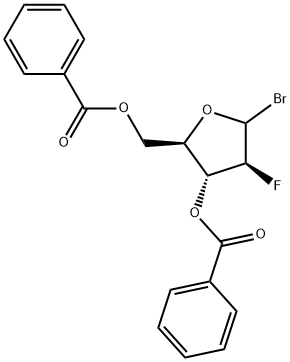
98855-71-1
2 suppliers
inquiry

97614-45-4
28 suppliers
$160.00/25mg
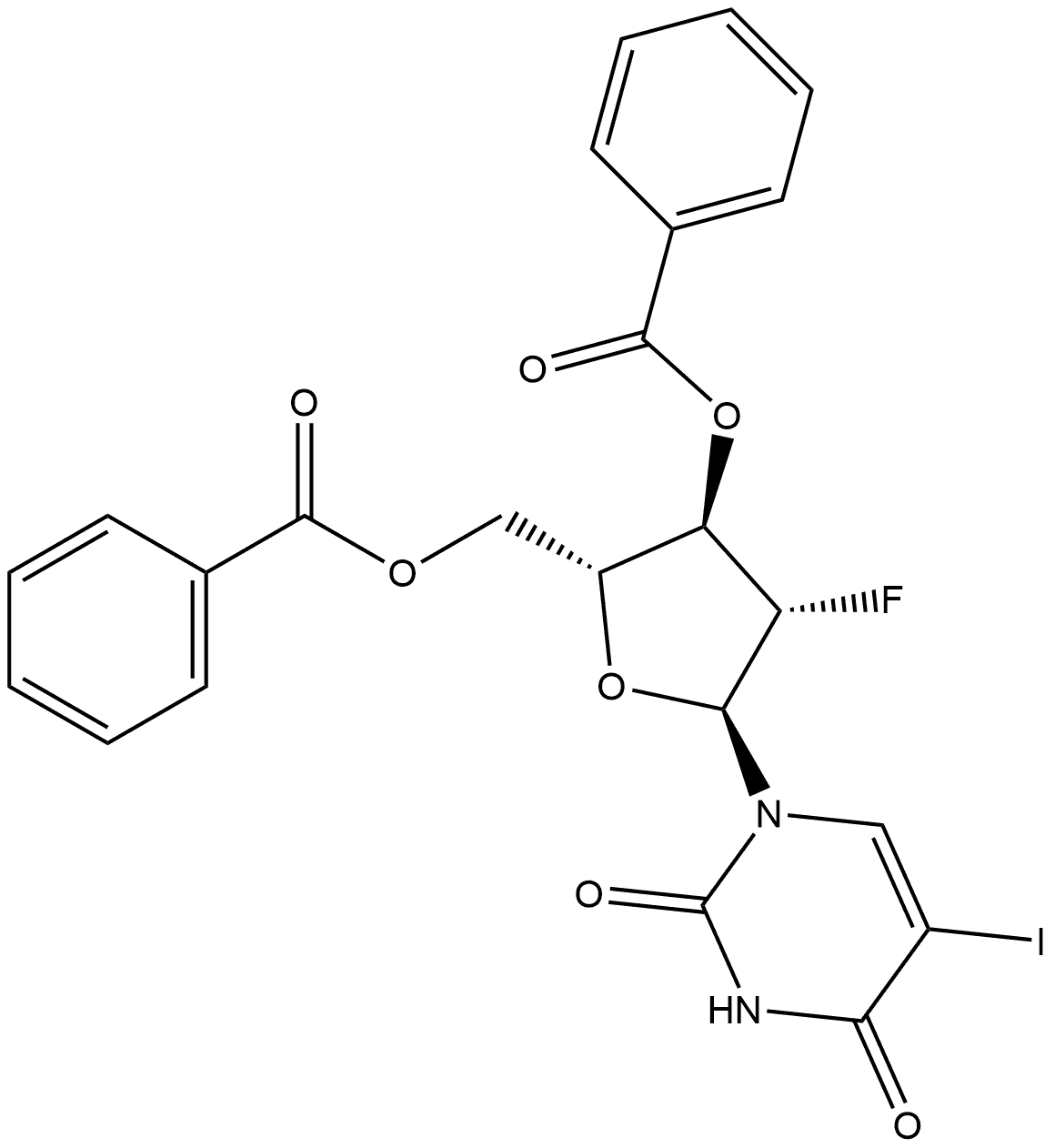
97614-46-5
0 suppliers
inquiry
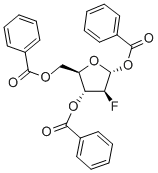
97614-43-2
331 suppliers
$5.00/250mg

97614-45-4
28 suppliers
$160.00/25mg
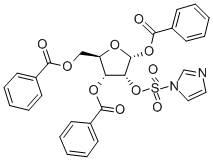
97614-42-1
41 suppliers
$55.00/10g

97614-45-4
28 suppliers
$160.00/25mg

97614-44-3
63 suppliers
$45.00/100mg

38953-72-9
3 suppliers
inquiry

97614-45-4
28 suppliers
$160.00/25mg

97614-46-5
0 suppliers
inquiry