| Identification | Back Directory | [Name]
TETRAKIS(ACETONITRILE)COPPER (I) HEXAFLUOROPHOSPHATE | [CAS]
64443-05-6 | [Synonyms]
tetrakis(acetonitrile)copper(I)
tetrakis(acetonitrile)copper hexa-fluorophosph
tetrakis(acetonitrile)copper(I) hexa-fluorophosph
Tetrakis(acetonitrle)copper(I) Hexafluorophosphate
Cuprous tetrakis(acetonitrile) hexafluorophosphate
Tetrakis(acetonitrile)copper [] hexafluorophosphate
Tetrakis(acetonitrile)copper [Ⅰ] hexafluorophosphate
Copper(I) tetrakis(acetonitrile) hexafluorophosphate
TETRAKIS(ACETONITRILE)COPPER (I) HEXAFLUOROPHOSPHATE
Tetrakis(acetonitrile)copper(I)hexafluorophosphate,98+%
Tetrakis(acetonitrile)copper(I) hexafluorophosphate 97%
Copper(1+), tetrakis(acetonitrile)-, (T-4)-, hexafluorophosphate(1-) | [Molecular Formula]
C8H12CuF6N4P | [MDL Number]
MFCD00064810 | [MOL File]
64443-05-6.mol | [Molecular Weight]
372.72 |
| Chemical Properties | Back Directory | [Melting point ]
160 °C (dec.)(lit.)
| [storage temp. ]
Inert atmosphere,Store in freezer, under -20°C | [form ]
solid | [color ]
white to light blue | [Sensitive ]
air sensitive, moisture sensitive |
| Questions And Answer | Back Directory | [Reaction]
- Useful catalyst for the Ullmann synthesis.
- Catalytic asymmetric [2,3]-Sigmatropic rearrangement of sulfur ylides.
- Precatalyst for enantioselective N–H insertion reactions with diazoesters and anilines.
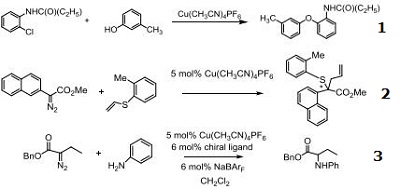
|
| Hazard Information | Back Directory | [Uses]
Tetrakis(acetonitrile)copper(I) hexafluorophosphate can be employed as a catalyst for the synthesis of triazolo[1,2-a]indazole-1,3,8-trione and 2H-indazolo[2,1-b]phthalazine-trione derivatives by the reaction of aryl aldehydes, dimedone and urazole or phthalhydrazide, respectively.
It can also be used as a copper precursor:
- For the formation of Cu2S/CdS nanorod junctions by cationic exchange process.
- To synthesize monometallic copper macrocyclic biquinazoline ligand [Cu(Mabiq)].
- To prepare the cuprous complex [Cu(2-iQBO)(POP)]PF6]; where 2-iQBO=2-(1′-isoquinolyl)benzoxazole and POP=bis[2-(diphenylphosphino)phenyl]ether, which can form luminescent pseudo-polymorphs.
- For the synthesis of [Cu(NHC)2]PF6 complexes [(NHC = N-heterocyclic carbene] which can be further used in the hydrosilylation of carbonyl compounds.
|
|
|