| Identification | Back Directory | [Name]
ETHYL 2-PHENYLACETOACETATE | [CAS]
5413-05-8 | [Synonyms]
a-acetyl-
99% Pure BMK
Bmk powder/oil
Ethyl 2-phenylacetoa
2-Phenylacetoacetate
BMK Glycidate BMK Powder
NEW BMK Glycidates Powder
ETHYL 2-PHENYLACETOACETAT
ETHYL 2-PHENYLACETOACETATE
Ethyl a-acetylbenzeneacetate
ethyl 2-phenyl-3-oxobutanoate
ETHYL 3-OXO-2-PHENYLBUTANOATE
ETHYL 2-PHENYLACETOACETATE bmk
BMK ETHYL 2-PHENYLACETOACETATE
Ethyl 2-phenylacetoacetate powder
Ethyl 2-phenylacetoacetate/New bmk
2-Phenylacetoacetic acid ethyl ester
ETHYL 2-PHENYLACETOACETATE/BMK POWDER
High purity Ethyl 2-phenylacetoacetate
2-Phenyl-3-oxobutyric acid ethyl ester
2-Phenyl-3-oxobutanoic acid ethyl ester
ETHYL 2-PHENYLACETOACETATE CAS 5413-05-8
BMK Powder ethyl 3-oxo-2-phenylbutanoate
Benzeneacetic acid, α-acetyl-, ethyl ester
Benzeneacetic acid, a-acetyl-, ethyl ester
BMK Glycidate ethyl 3-oxo-2-phenylbutanoate
(2R)-3-oxo-2-phenylbutanoic acid ethyl ester
ETHYL 2-PHENYLACETOACETATE Basic information
5413-05-8 2-Phenylacetoacetic acid ethyl ester
high quality 2-PHENYLACETOACETATE CAS 5413-05-8
Benzeneacetic acid, .alpha.-acetyl-, ethyl ester | [EINECS(EC#)]
226-500-0 | [Molecular Formula]
C12H14O3 | [MDL Number]
MFCD00040490 | [MOL File]
5413-05-8.mol | [Molecular Weight]
206.24 |
| Chemical Properties | Back Directory | [Melting point ]
140-144°C/10mm | [Boiling point ]
140-144°C 10mm | [density ]
1,085 g/cm3 | [refractive index ]
1.5130 | [Fp ]
140-144°C/10mm | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [pka]
10.69±0.46(Predicted) | [BRN ]
1912655 | [InChI]
InChI=1S/C12H14O3/c1-3-15-12(14)11(9(2)13)10-7-5-4-6-8-10/h4-8,11H,3H2,1-2H3 | [InChIKey]
PWRUKIPYVGHRFL-UHFFFAOYSA-N | [SMILES]
C(C(=O)C)(C(=O)OCC)C1C=CC=CC=1 |
| Hazard Information | Back Directory | [Uses]
Ethyl 2-Phenylacetoacetate is used in preparation of iridium polysubstituted quinoline diketonate complex and application as OLED. | [Synthesis]
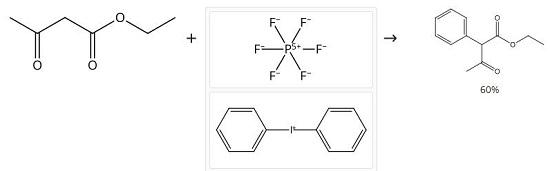
A flame-dried flask was charged with 10 mmol (1 equiv) of sublimed potassium tert-butoxide in anhydrous DMF (50 mL) at room temperature under argon. Then, 10 mmol (1 equiv) of freshly distilled EAA was added to the reaction mixture and stirred for 30 min at 0 °C, followed by dropwise addition of diaryliodonium salt (4 mmol, 0.4 equiv to EAA) in 10 mL of DMF. Reaction was left stirring at room temperature for the time mentioned in the table. After confirming complete consumption of iodonium salt (by LCMS), to the reaction mixture was added 1 M HCl in one portion to bring the pH to around 5.0. The crude was extracted with diethyl ether until the aqueous layer was devoid of product. The organic layer was dried over sodium sulfate, and solvent was removed in vacuo. The product was purified by flash column chromatography (0.5-2% of hexane in ethyl acetate). Ethyl 2-phenylacetoacetate 1H NMR (500 MHz, CDCl3) δ 13.13 (s, 0.3H), 7.41-7.27 (m, 4H), 7.18-7.13 (m, 1H), 4.69 (s, 0.7H), 4.27-4.15 (m, 2H), 2.19 (s, 2H), 1.86 (s, 1H), 1.28 (t, J = 7.1 Hz, 2H), 1.18 (t, J = 7.1 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 201.7, 174.0, 172.7, 168.6, 135.4, 132.8, 131.4, 129.4, 129.0, 128.4, 128.1, 127.0, 104.5, 65.9, 61.8, 60.8, 28.9, 20.0, 14.3, 14.2. HRMS (ESI-TOF) m/z: [M + H]+ calcd for C12H14O3 207.1016; found 207.1018.
|
|