| Identification | Back Directory | [Name]
1-Methyl-5-amino-1H-benzimidazole-2-butanoic acid ethyl ester | [CAS]
3543-73-5 | [Synonyms]
5-AMino-1-Methyl-
Bendamustine Intermediate
1-Methyl-5-amino-1H-benzimidazole-2
BendaMustine hydrochloride interMediate
endamustine Dideschloroethyl Ethyl Ester
BendaMustine Dideschloroethyl Ethyl Ester
Bendamustine hydrochloride Intermediates 2
4-(5-amino-1-methyl-1H-benzimidazol-2-yl)butanoate
Ethyl 5-amino-1-methyl-1H-benzimidazole-2-butanoate
ethyl 4-(5-amino-1-methylbenzimidazol-2-yl)butanoate
5-amino-1-methyl-2-Benzimidazolebutyric acid ethyl ester
ETHYL 4-(5-AMINO-1-METHYL-1H�BENZIMIDAZOL-2-YL) BUTANOATE
5-(5-amino-1-methyl-2-benzimidazolyl)-1-methoxy-2-pentanone
ethyl 4-(5-aMino-1-Methyl-1H-1,3-benzodiazol-2-yl)butanoate
1-Methyl-5-aMino-1H-benziMidazole-2-butanoic acid ethyl este
Ethyl 4-(5-amino-1-methyl-1H-1,3-benzimidazol-2-yl)butanoate
Ethyl-4-(5-aMino-1-Methy-1H-benzo[d] iMidazol-2-yl)butanoate
ethyl 4-(5-amino-1-methyl-1H-benzo[d]imidazol-2-yl)butanoate
1-Methyl-5-amino-1H-benzimidazole-2-butanoic acid ethyl ester
5-AMINO-1-METHYL-1H-BENZIMIDAZOLE-2-BUTANOIC ACID ETHYL ESTER
Ethyl4-(5-amino-1-methyl-1H�benzo[d]imidazole
-2-yl)butanoate
4-[5-AMino-1-MethylbenziMidazol-2-yl]butanoic acid ethyl ester
1H-Benzimidazole-2-butanoic acid, 5-amino-1-methyl-, ethyl ester
4-(5-Amino-1-methyl-1H-benzoimidazol-2-yl)-butyric acidethylester
Bendamustine Hydrochloride Intermediate 3543-73-5 API Manufacturer
4-(5-aMino-1-Methyl-1H-benzoiMidazol-2-yl)-butyric acid ethyl esterCAS | [EINECS(EC#)]
680-673-0 | [Molecular Formula]
C14H19N3O2 | [MDL Number]
MFCD09840909 | [MOL File]
3543-73-5.mol | [Molecular Weight]
261.32 |
| Chemical Properties | Back Directory | [Melting point ]
130-135°C | [Boiling point ]
471.6±25.0 °C(Predicted) | [density ]
1.21±0.1 g/cm3(Predicted) | [storage temp. ]
Keep in dark place,Inert atmosphere,2-8°C | [solubility ]
Chloroform (Slightly), Ethyl Acetate (Slightly) | [form ]
Solid | [pka]
6.73±0.10(Predicted) | [color ]
Off-White to Pale Beige | [InChI]
InChI=1S/C14H19N3O2/c1-3-19-14(18)6-4-5-13-16-11-9-10(15)7-8-12(11)17(13)2/h7-9H,3-6,15H2,1-2H3 | [InChIKey]
JUMGOLYNZBZPKE-UHFFFAOYSA-N | [SMILES]
C1(CCCC(OCC)=O)N(C)C2=CC=C(N)C=C2N=1 |
| Hazard Information | Back Directory | [Chemical Properties]
Brown Solid | [Uses]
Bendamustine | [Synthesis]
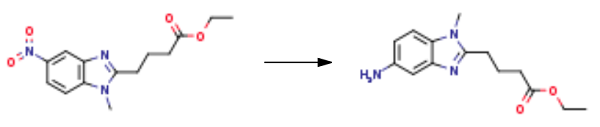
In the hydrogenation reaction kettle by adding 50 g 1 - methyl -5 - nitro - 1H - benzimidazole -2 - butyric acid ethyl ester, 2.5 g 5% palladium/carbon, 1.3 L methanol, 475 ml ethyl acetate, hydrogen pressure control in the 0.1 - 0.2 mpa, reaction solution is 25 °C reaction 15 hours, TLC monitoring endpoint of the reaction to the reaction is complete. The fluid in a 35 °C decompression filter collects the filtrate; the filtrate is concentrated under reduced pressure to dry after adding 75 ml ethyl acetate, 70 °C stirring for 0.5 hours. After that, the mixture was to 5 °C stirring for 0.5 hours, for 5 °C standing crystallization for 2 hours, filtering, washing the filter cake with acetic acid ethyl ester, filtration cake at 50 °C decompression drying for 5 hours to afford 1-Methyl-5-amino-1H-benzimidazole-2-butanoic acid ethyl ester 35 g, yield 77.8%.
|
|
|