| Identification | Back Directory | [Name]
(1-ETHOXYCYCLOPROPOXY)TRIMETHYLSILANE | [CAS]
27374-25-0 | [Synonyms]
(1-ETHOXYCYCLOPROPOXY)TRIMETHYLSILANE
1-ETHOXY-1-TRIMETHYLSILOXYL CYCLOPROPANE
1-Ethoxy-1-(trimethylsiloxy)cyclopropane
(1-ETHOXYCYCLOPROPOXY)TRIMETHYLSILANE 99%
[(1-ETHOXYCYCLOPROPYL)OXY]TRIMETHYLSILANE
Trimethyl[(1-ethoxycyclopropyl)oxy]silane
(1-Ethoxycyclopropoxy)trimethylsilane,99%
CYCLOPROPANONE ETHYL TRIMETHYLSILYL ACETAL
1-(Trimethylsilyloxy)-1-ethoxycyclopropane
1-Ethoxy-1-(trimethylsilyloxy)cyclopropane
2- 1-Ethoxy-1-trimethylsiloxylcyclopropane
1-Ethyloxy-1-(trimethylsilyloxy)cyclopropane
SILANE, [(1-ETHOXYCYCLOPROPYL)OXY]TRIMETHYL-
(1-Ethoxycyclopropoxy)trimethylsilane ,98.5%
(1-Ethoxycyclopropoxy)triMethylsilane, 99% 5GR
(1-Ethoxycyclopropoxy)triMethylsilane, 99% 25GR
(1-Ethoxycyclopropoxy)trimethylsilaneCyclopropanone ethyl trimethylsilyl acetal | [Molecular Formula]
C8H18O2Si | [MDL Number]
MFCD00074986 | [MOL File]
27374-25-0.mol | [Molecular Weight]
174.31 |
| Chemical Properties | Back Directory | [Appearance]
clear colorless liquid | [Boiling point ]
50-53 °C22 mm Hg(lit.)
| [density ]
0.867 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.407(lit.)
| [Fp ]
79 °F
| [storage temp. ]
Flammables area | [solubility ]
insol H2O. | [form ]
Liquid | [color ]
Clear colorless | [Specific Gravity]
0.867 | [Hydrolytic Sensitivity]
7: reacts slowly with moisture/water | [BRN ]
1924720 | [InChIKey]
BZMMRNKDONDVIB-UHFFFAOYSA-N | [CAS DataBase Reference]
27374-25-0 |
| Hazard Information | Back Directory | [Chemical Properties]
clear colorless liquid | [Physical properties]
bp 50–53 °C/22 mmHg. | [Uses]
The 1-Ethoxy-1-(trimethylsilyloxy)cyclopropane can be used as preparation of 3-metallopropionates; metal homoenolate
precursor; γ-hydroxy esters; cyclopentenones; 3-aminopropio�nates; cyclopropylamine formation; 1-aminocyclopropane�carboxylic acids and 1-aminocyclopropanephosphonic acids;
β- and γ-amino acids.
The 1-Ethoxy-1-(trimethylsilyloxy)cyclopropane is widely used in various reactions. Cyclization of optically pure β-halo esters gives cyclopropanone acetals enantiomerically pure at C-2 and a 1:1 diastereomeric mixture at C-1.
Use of Cyclopropanone Hemiacetals. Heating cyclopropanone hemiacetal at 100°Cin an aqueous buffer provides the cyclopropanone hydrate. It also serves as a source of homoenolate radical species with a catalytic amount of AgNO3.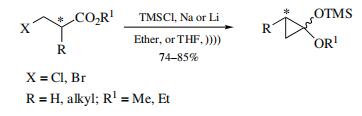
| [Preparation]
For the synthesis of the parent and the
2-monoalkyl-substituted compounds, reduction of ethyl 3-
chloropropionate with sodium–potassium alloy in the presence
of chlorotrimethylsilane in ether. A recent modification using ultrasound irradiation is much more convenient and more
widely applicable. Other substituted derivatives are prepared
by cyclopropanation of alkyl silyl ketene acetals with the
Furukawa reagent (diiodomethane/diethylzinc). |
|
|