| Identification | Back Directory | [Name]
METHYL 4-OXOTETRAHYDROTHIOPHENE-3-CARBOXYLATE | [CAS]
2689-68-1 | [Synonyms]
AKOS 92615
methyl tetrahydro-4-oxo-3-thenoate
4-CARBOMETHOXYTETRAHYDRO-3-THIOPHENE
4-CARBOMETHOXYTETRAHYDRO-3-THIOPHENONE
METHYL 3-OXOTETRAHYDROTHIOPHENE4-CARBOXYLATE
METHYL 4-OXOTETRAHYDROTHIOPHENE-3-CARBOXYLATE
Methyl 4-oxotetrahydrothiophene-3-carboxylate ,97%
Methyl 4-oxotetrahydrothiophene-3-carboxylate, 95+%
tetrahydro-4-oxo-3-thiophenecarboxylicacimethylester
Methyl 4-oxotetrahydrothiophene-3-carboxylate , Tech.
4-Oxo-Tetrahydro-Thiophene-3-CarboxylicAcidMethylEster
Tetrahydro-4-oxo-3-thiophenecarboxylic acid methyl ester
Methyl 4-oxotetrahydrothiophene-3-carboxylate, technical
METHYL 4-OXOTETRAHYDROTHIOPHENE-3-CARBOXYLATE ISO 9001:2015 REACH
3-(Methoxycarbonyl)-4-oxotetrahydrothiophene, 3-(Methoxycarbonyl)-4-oxothiolane | [EINECS(EC#)]
220-256-9 | [Molecular Formula]
C6H8O3S | [MDL Number]
MFCD00052381 | [MOL File]
2689-68-1.mol | [Molecular Weight]
160.19 |
| Hazard Information | Back Directory | [Chemical Properties]
METHYL 4-OXOTETRAHYDROTHIOPHENE-3-CARBOXYLATE is White solid
| [Uses]
METHYL 4-OXOTETRAHYDROTHIOPHENE-3-CARBOXYLATE is used in the preparation of a new class of neuroleptic agents.
| [Synthesis]
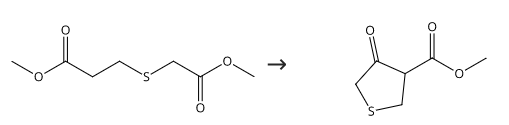
2,3,4,5-Tetrahydro-4-oxo-3-thiophene carboxylic acid, methyl ester (L75a): 25.3 g (1.1 mol) of sodium were implemented in 190 mL abs. methanol to methylate. To methylate solution 70 g (0.360 mol) (L74) was added dropwise at the boiling point and the reaction mixture was heated for 45 min under reflux. The cooling mixture was poured onto a mixture of 500 mL ice and 300 mL water, acidified with 100 mL conc. HCl and the oily precipitating product was brought to crystallize under cooling with ice/sodium chloride bath. The crystals were sucked off, resumed in 100 mL methylene chloride and dried over sodium sulfate and evaporated. There were obtained 16.7 g of pale beige crystals. The aqueous phase was extracted three times with 100 mL of methylene chloride, the organic phase dried over sodium sulfate and evaporated. 30 g of crystalline product Methyl 4-oxotetrahydrothiophene-3-carboxylate was obtained. Yield: 46.7 g of light beige crystals (81 % of theory). mp 29-31 degC. TLC: Bz:MeOH:AcOH = 45:8:4, Rf = 0.75.
 |
|
|