| Identification | Back Directory | [Name]
NOCARDAMINE | [CAS]
26605-16-3 | [Synonyms]
Nocardamin
NOCARDAMINE
PROFERRIOXAMIN E
deferrioxamine E
Desferrioxamine E
Desferri-ferrioxamine E
Nocardamine (Deferrioxamine E)
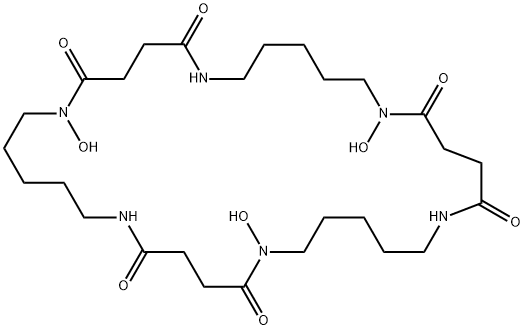
1,12,23-Trihydroxy-1,6,12,17,23,28-hexaazacyclotritriacontane-2,5,13,16,24,27-hexone
6,17,28-Trihydroxy-1,6,12,17,23,28-hexaazacyclotritriacontane-2,5,13,16,24,27-hexaone
1,6,12,17,23,28-Hexaazacyclotritriacontane-2,5,13,16,24,27-hexone, 1,12,23-trihydroxy- | [Molecular Formula]
C27H48N6O9 | [MDL Number]
MFCD08457937 | [MOL File]
26605-16-3.mol | [Molecular Weight]
600.7 |
| Chemical Properties | Back Directory | [Melting point ]
192-195° | [density ]
1.162±0.06 g/cm3(Predicted) | [storage temp. ]
-20°C | [solubility ]
Soluble in DMSO (5 mg/ml) | [form ]
solid | [pka]
8?+-.0.20(Predicted) | [color ]
Off-white | [Stability:]
Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| Hazard Information | Back Directory | [Description]
Nocardamine is a ferrioxamine siderophore that has been found in Streptomyces and has diverse biological activities. It chelates iron in a chrome azurol S assay (IC50 = 9.9 μM). Nocardamine inhibits M. smegmatis and M. bovis biofilm formation (MIC = 10 μM for both), an effect that can be reversed by iron. It is cytotoxic to T47D, SK-MEL-5, SK-MEL-28, and RPMI-7951 cancer cells (IC50s = 6, 18, 12, and 14 μM, respectively). Nocardamine also induces morphological changes in BM-N4 insect cells. | [Uses]
Nocardamine is a cyclic siderophore. | [Definition]
ChEBI: A cyclic hydroxamic acid siderophore that is produced by several bacterial species and exhibits antitumour activity. | [storage]
+4°C | [References]
Normant et al. (2020), Nocardamine-Dependent Iron Uptake in Pseudomonas aeruginosa: Exclusive Involvement of the FoxA Outer Membrane Transporter; ACS Chem. Biol. 15 2741
Ueki et al. (2009), Nocardamin Production by Streptomyces avermitilis; Actinomycetologica 23 34
Yan et al. (2021), Ferroptosis: mechanisms and links with diseases; Signal Transduct. Target Ther. 6 49
Kalinovskaya et al. (2011), Marine isolate Citricoccus sp. KMM 3890 as a source of a cyclic siderophore nocardamine with antitumor activity; Res. 166 654
Mahmud et al. (2022), Bioactivities and Mode of Actions of Dibutyl Phthalates and Nocardamine from Streptomyces sp. H11809; Molecules 27 2292 |
|
| Company Name: |
BOC Sciences
|
| Tel: |
1-631-485-4226; 16314854226 |
| Website: |
https://www.bocsci.com |
| Company Name: |
Energy Chemical
|
| Tel: |
021-58432009 400-005-6266 |
| Website: |
http://www.energy-chemical.com |
| Company Name: |
|
| Tel: |
4008-099-669 |
| Website: |
http://www.yuhua99.com/ShowSupplierProductsList112975/0.htm |
|