| Identification | Back Directory | [Name]
1H-1,2,4-TRIAZOLE-1-CARBOXAMIDINE MONOHYDROCHLORIDE | [CAS]
19503-26-5 | [Synonyms]
NOLA-010
1H-1,2,4-TRI
TIMTEC-BB SBB004089
Methotrexate (Abitrexate)
1-CARBAMIDINO-1,2,4-TRIAZOLE
1H-1,2,4-Triazole-1-CarboxaMidine
1-AMIDINO-1,2,4-TRIAZOLE HYDROCHLORIDE
1H-1,2,4-TRIAZOLE-1-CARBOXAMIDINE MONOHY
1-amidino-1h-1,2,4-triazole hydrochloride
1-Amidino-1,2,4-triazole monohydrochloride
(1H)-1,2,4-Triazole-1-carboxamidineHClpurum
1-Carbamimidoyl-1,2,4-triazole Hydrochloride
[1,2,4]Triazole-1-carboxaMidine Hydrochloride
1,2,4-Triazole-1-carboximidamide Hydrochloride
1h-1,2,4-triazole-1-carboxamidinehydrochloride
1H-1,2,4-triazole-1-carboxiMidaMide hydrochloride
1H-1,2,4-TRIAZOLE-1-CARBOXAMIDINE MONOHYDROCHLORIDE
1H-1,2,4-Triazole-1-carboxaMidine Monohydrochloride ,98%
1H-1,2,4-Triazole-1-carboximidamide, hydrochloride (1:1)
(1H)-1,2,4-Triazole-1-carboxamidinemonohydrochloridepurum
1H-1,2,4-Triazole-1-carboxamidine monohydrochloride ,98.5%
1H-1,2,4-TRIAZOLE-1-CARBOXAMIDINE MONOHY DROCHLORIDE PURUM 98+%
1H-1,2,4-Triazole-1-carboxamidine hydrochloride≥ 98%(Titration) | [EINECS(EC#)]
606-331-2 | [Molecular Formula]
C3H6ClN5 | [MDL Number]
MFCD03095468 | [MOL File]
19503-26-5.mol | [Molecular Weight]
147.57 |
| Chemical Properties | Back Directory | [Melting point ]
215-220 °C | [storage temp. ]
Inert atmosphere,Room Temperature | [Water Solubility ]
Soluble in water | [form ]
powder to crystal | [color ]
White to Almost white | [BRN ]
9500271 | [InChI]
InChI=1S/C3H5N5.ClH/c4-3(5)8-2-6-1-7-8;/h1-2H,(H3,4,5);1H | [InChIKey]
JDDXNENZFOOLTP-UHFFFAOYSA-N | [SMILES]
C(N1N=CN=C1)(N)=N.Cl | [CAS DataBase Reference]
19503-26-5 |
| Hazard Information | Back Directory | [Chemical Properties]
White crystalline | [Uses]
[1,2,4]Triazole-1-carboxamidine is an intermediate used in the synthesis of peramivir (P285500), a neuraminidase inhibitor developed as an antiviral agent for the treatment of influenza. | [Synthesis]
1H-1,2,4-Triazole-1-carboxamidine hydrochloride is prepared by the reaction of dicyandiamide and 1,2,4-Triazole. The steps are as follows:
Dissolve the compound (19.7 g, 0.29 mol) in 20 ml of tetrahydrofuran, and the temperature was raised to 70℃. A solution of dicyandiamide (20 g, 0.24 mol) was added and a solution of 4 mol / L HCl in tetrahydrofuran 27 g, 0.26 mol) at 70℃ for 2 h, the temperature was removed and cooled to room temperature. The solid was removed by filtration and 26.0 g was removed by filtration. The solvent was dried in vacuo at 45℃ to give a colorless oil which was recrystallized from methanol: dichloromethane = 2: 1 Of the mixed solution 50ml recrystallization, cooling crystallization, suction filter to get 6.2g solid, drying a total of 30.1g white powder. Yield 90.3%, purity 99,8%.
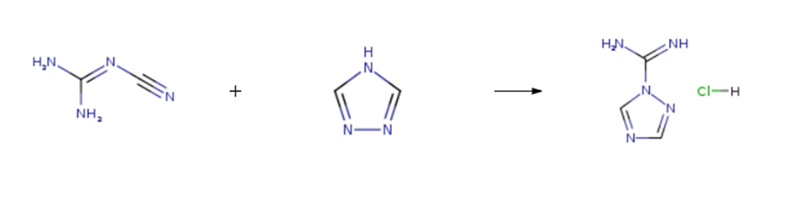 |
|
|